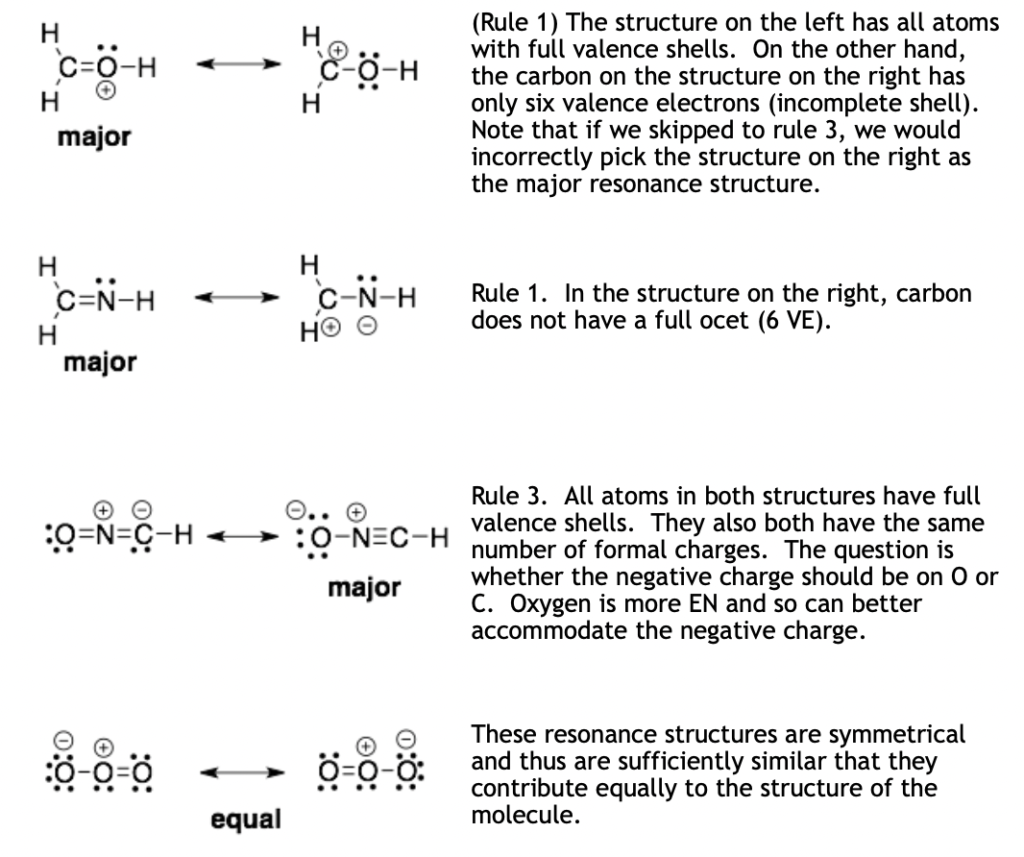
Assigning the major resonance structure
Introduction:
In some cases, resonance structures are sufficiently similar that they contribute equally to the structure of a molecule. In other cases, one resonance structure will provide a more accurate description of the molecule and thus is assigned as the major resonance structure. Most textbooks are clear on what types of characteristics can make one resonance structure better than another. However, what order these rules are applied is a subject for some debate. The reason is that these rules are all manmade constructs that were developed to help teach and explain the observed structures and reactivites of organic molecules. I have picked a hybrid set of rules that are easy to apply and should identify the major resonance structure in most situations.
How to:
Compare two resonance structures and apply the following rules in order, starting with rule 1 and ending at rule 3. Stop when you find a difference between the two structures.
- The major resonance structure will have more atoms with full valence shells.
- The major resonance structure will have fewer formal charges or positive and negative charges that are closer to each other.
- The major resonance structure will have the formal charges on atoms of matching electronegativity (EN). Negative charges on more EN atoms and positive charges on less EN atoms.
Examples: