Moving electrons in resonances structures
Introduction:
Resonance structures differ only in how the electrons are distributed between the atoms. Therefore, if we shift around the electrons in one resonance structure, then it can be transformed into other resonance structure.
How to:
Organic chemists use a curved arrow formalism to show the movement of a pair of electrons. The three most fundamental curved arrow moves are:
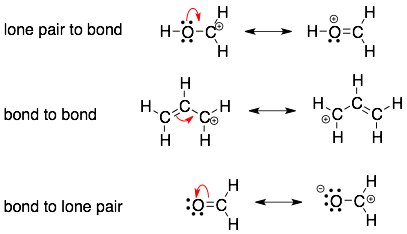
Note: The electrons do not jump from one atom to another. The electrons stay anchored to one atom. In the top example, the lone pair on oxygen forms a new bond with the adjacent carbon atoms. However, the electrons are still part of the octet on oxygen. Therefore, the one move that we cannot make is to move a lone pair on one atom to a lone pair on an adjacent atom because then the electrons will be effectively hopping from one atom to the next. Electrons can be moved from one atom to another atom but it takes multiple curved arrows like in the examples below.
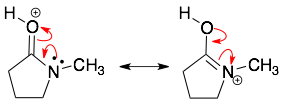
HINT 1: It is all about the electrons. The curved arrows show the movement of electrons (which forms and breaks bonds). The curved arrows do not show the motion of atoms or charges. Sometimes, it looks like the curved arrows are moving charges or atoms around, but what is really happening is that after the electrons move around different atoms may gain or lose their formal charges. In each of the examples above, notice that the backside and head of the arrow always points to a source of electrons (a lone pair or bond).
HINT 2: Multiple curved arrows point will flow in the same direction. This is because electrons will flow. The arrows should not point away from each other or point toward each other.
HINT 3: Electrons should flow toward positive charges and away from negative charges. Again, examine the examples above that have formal charges. The arrows are moving away from negative charges and toward positive charges.
HINT 4: Beware of exceeding the octet rule. A common error is to move electrons to form an invalid Lewis Structure such as in the example below. The carbon on the right is a CH3 (the 3 H’s are implicit). So if we make a double bond to it (=CH3) then the carbon will have five bonds and ten valence electrons!!
