Predicting solubility trends
Introduction:
The key is to understand why a molecule might want to dissolve in a solvent (or if two solvents are miscible)? Condensed matter has cohesive molecule-molecule interactions that keep them together as a solid (or liquid). So why would it want to break these interactions?
The answer is that a solvent will dissolve a molecule if the solvent can form equally strong or stronger molecule-solvent interactions than the molecule-molecule interactions. In other words, does the molecule like the solvent more than it likes itself?
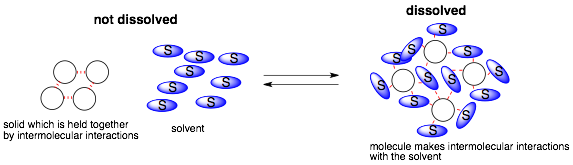
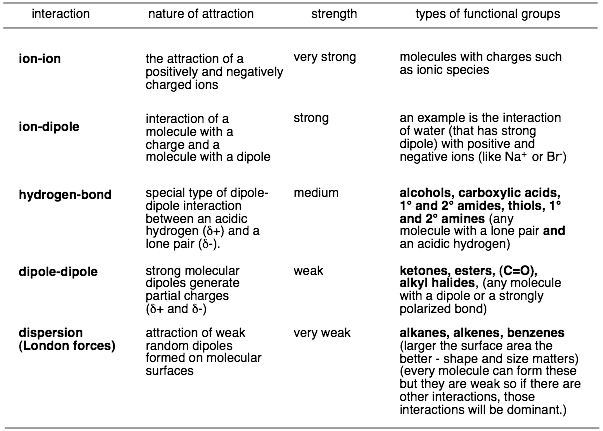
In order to answer this question, we have to be able to predict what interactions a molecule can make with itself or a solvent and what the relative strengths of these interactions are. Below are a list of intermolecular interactions and their relative strengths.
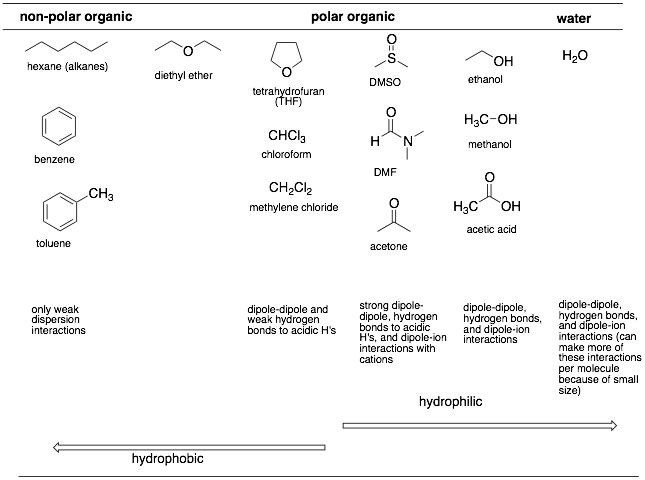
We also need to classify the common solvents by their ability to make the above interactions. There are three general classes of solvents but the overall scale is a continuum. Notice that as we go toward water, we can make more an more of the strong intermolecular forces. And on the left-hand side, the non-polar organic solvents can only make weak intermolecular (dispersion) interactions.
How to:
So the simplest prediction that we can make is: will a molecule dissolve in non-polar organic solvent (hydrophobic) or in water (hydrophilic).
Molecules that have strong cohesive molecule-molecule interactions such as ions (charged molecules and salts) or strong hydrogen bonds (alcohols, carboxylic acids etc) will not dissolve in non-polar solvents because the hydrophobic solvents cannot form strong interactions with these molecules. Water, on the hand, can form strong ion-dipole and hydrogen bonding interactions and thus can dissolve molecules that are held together by strong cohesive interactions.
Non-polar organic solvents will dissolve hydrophobic molecules, which are molecules that do not have polar groups like ions and hydrogen bonding functionality (alcohols, carboxylic acids. The reason is that they are all in the ‘weakly attractive club’ so the solvent can break the weak molecule-molecule dispersion interactions.
One interesting question is why doesn’t water dissolve hydrophobic molecules (and solvents)? The answer is that, yes, water can form cohesive interactions with hydrophobic molecules but that water can form much stronger cohesive interactions with itself. So water, effectively, excludes the ‘unattractive’ hydrophobic molecules from its ‘clique’ of ‘attractive molecules’ like itself.