Find the most acidic proton in a molecule
Introduction:
Most molecules contain different types of protons (bonded hydrogens). However, when acting as acids, only the most acidic proton will participate in the acid-base reaction. Therefore, it is important to be able to identify the most acidic proton in a molecule.
How to:
There are three general methods to estimate the acidity of a proton:
1) Use the table of common acids that we learned in class and is in your book (Table 3.1, inside front cover of the book), and try to either find the same acidic proton or similar one in the table.
2) Identify which functional group the proton is a part of like: alkane, alcohol, carboxylic acid, protonated amine, etc… Make sure that the proton in question is actually part of the functional group and is not simply attached to atoms that are attached to the functional group. Here is a short list of acidities of common functional groups in organic chemistry (Note: that the pKa’s are an approximate value for that functional group.)
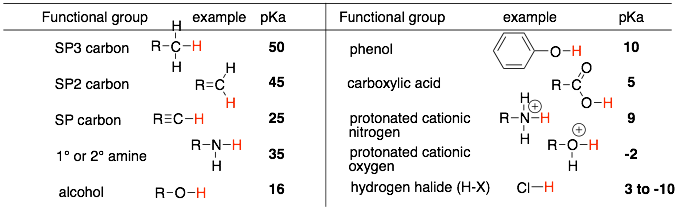
3) Use the trends we learned in class to compare the stabilities of the conjugate bases (the acid with the proton taken away) of the acids.
Hint: The most stable conjugate base will be the strongest acid.
Examples
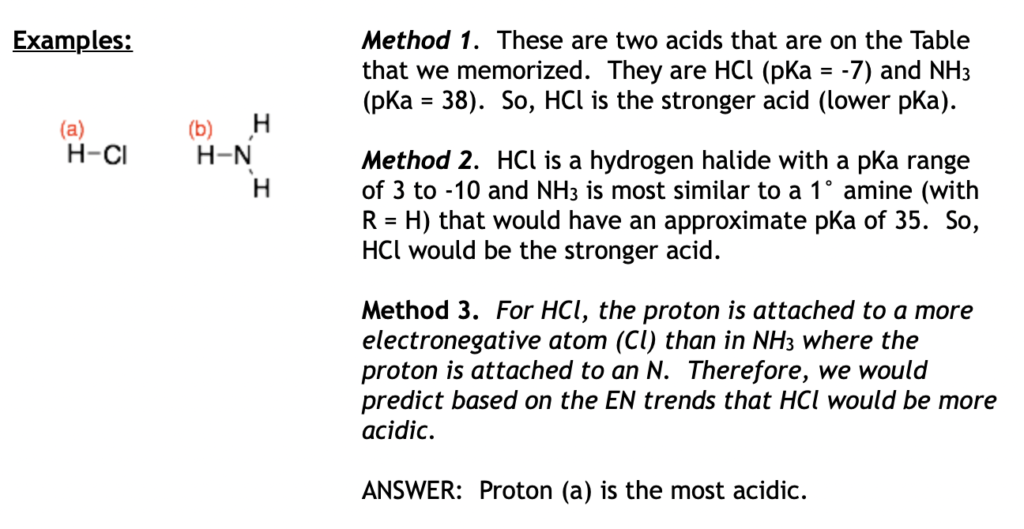
Hint: For molecules with lots of different types of protons, I think method two is the easier to apply because it is the fastest.


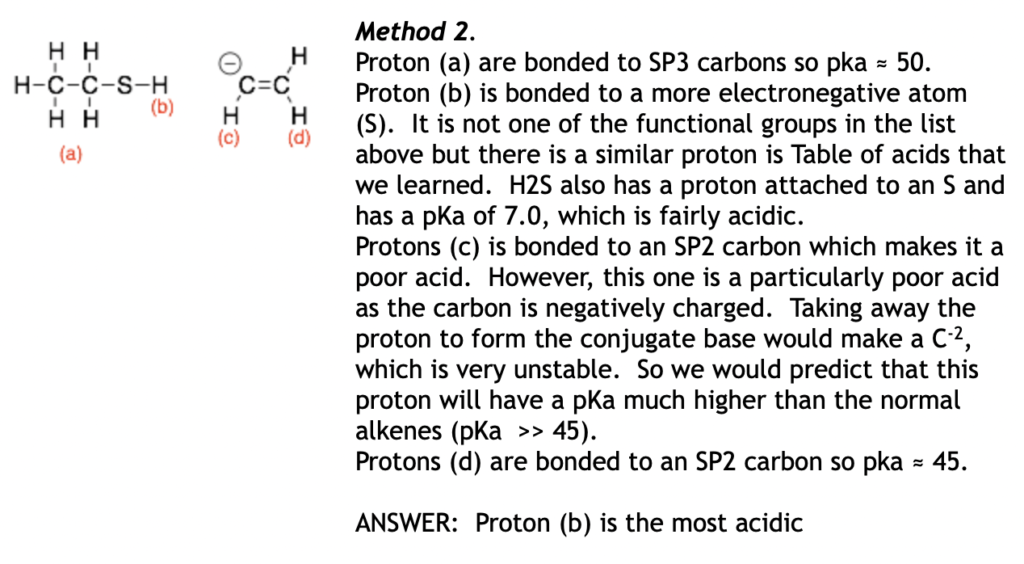
Hint: Protons on carbon are rarely the most acidic because carbon is not very electronegative. Look at protons on more electronegative atoms first O, N, S etc first.