Name alkanes and haloalkanes
Introduction:
The IUPAC (International Union of Pure and Applied Chemistry) sets out a systematic set of rules to name every possible molecule. The rules were written so 1) each molecule will have a unique name and 2) by following the rules, everyone would come up with the same unique name for a molecule. This ensure that chemists all over the world will ‘speak’ the same language. This tutorial will focus on how to name non-cyclic alkanes and haloalkanes. The rules of molecules with rings are in the next section.
How to:
Follow the instructions below to name non-cyclic alkanes and haloalkanes
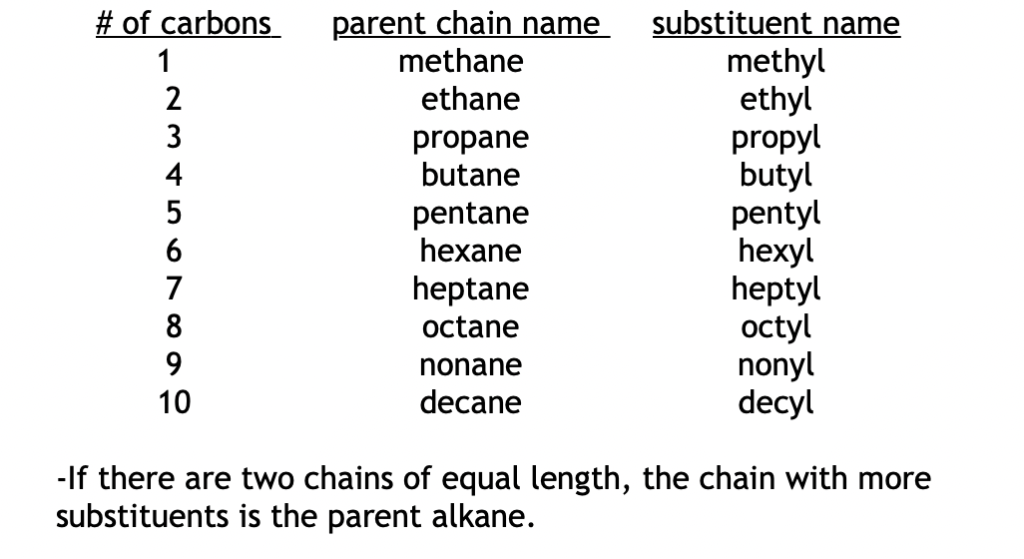
1) Find the longest continuous linear chain of carbons. Name this parent chain using the list below. Carbons or halogens that are not part of the main chain are substituents.
2) Number the parent chain from 1 to n. Start numbering from the end of the parent chain that is closest to the first substituent.
a)if both ends are the same distance from the first substituent, chose the end that is closest to the second substituent
b) if everything else is the same, pick the end that with the first substituent that is first alphabetically
3) Name all groups (substituents) attached to the parent chain. Carbon substituents are named using the same terms as the parent chain except the -ane ending is changed to -yl.
substituent with one carbon –> methyl
substituent with four carbons –> butyl
Halogen substituents (F, Cl, Br, I) are named: fluoro, chloro, bromo, iodo.
4) Group substituents that repeat together using the prefixes: di-, tri-, tetra- etc.
i.e. dimethyl or tetrachloro or triethyl
5) List the substituents in alphabetical order ignoring the prefixes.
i.e. ethyl before methyl or ethyl before dimethyl
iso and neo are not prefixes (so isopropyl comes before methyl)
6)Write out the name in the format:
#,#-prefixsubstituent-#-prefixsubstituentparentchain
The #-signs are the number of the carbon on the parent chain where the substituent is attached.
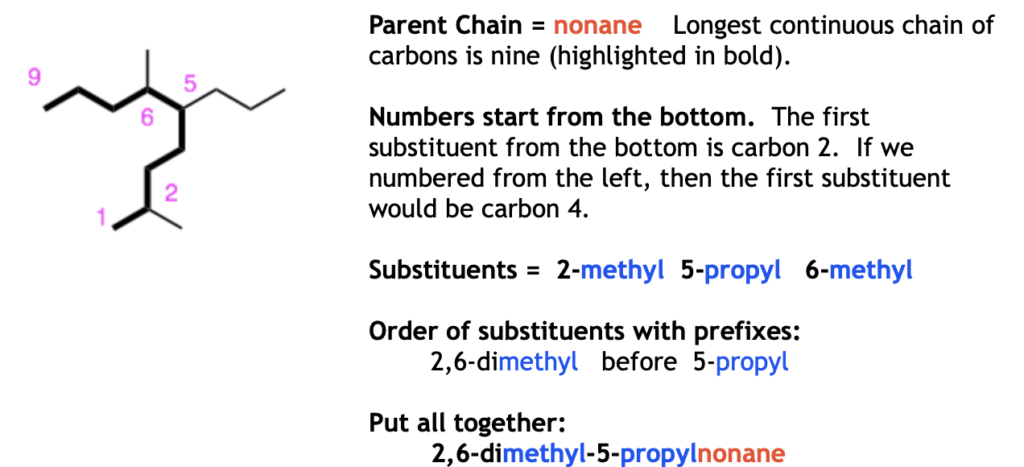
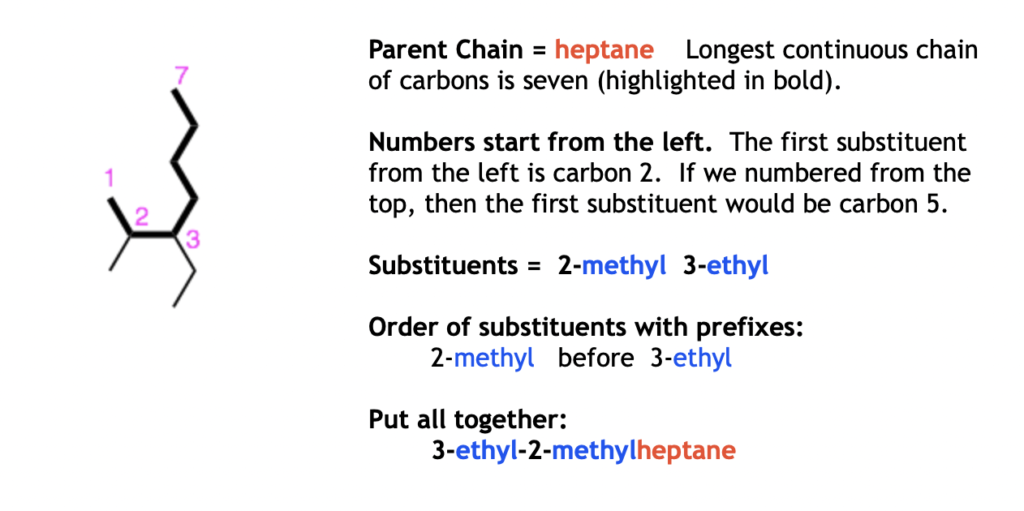
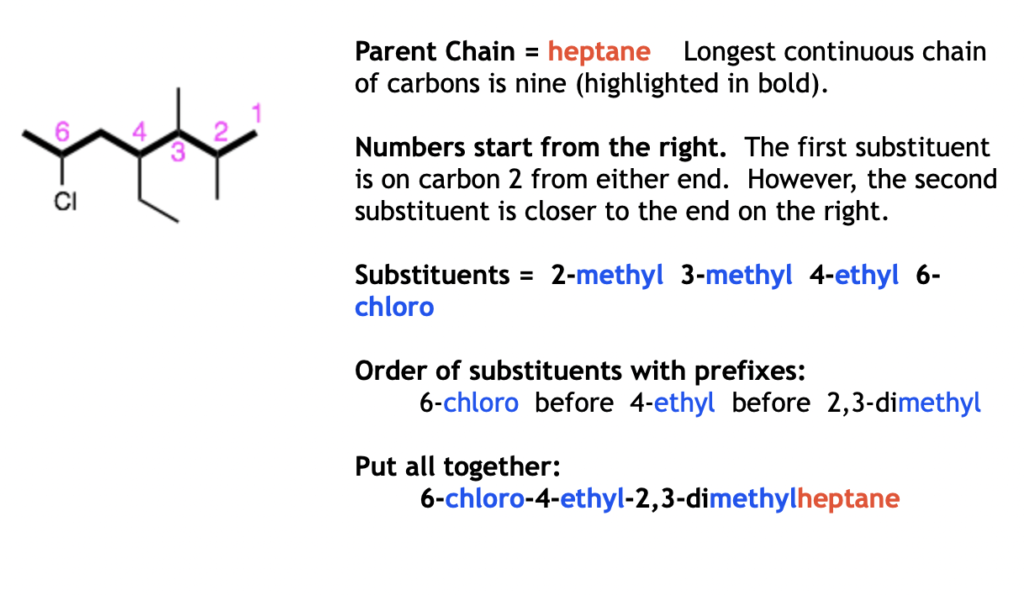
Examples: