Interpret and Draw Newman Projections
Introduction:
When drawing bond-line drawings on a flat piece of paper, it is hard to depict and visualize the process of rotating around a single bond. This is why the Newman projection is useful but it takes some getting used to. It is an odd point of view looking down the end of a bond, and so it is very easy to add or lose carbons.
How to:
In the Newman projection, we are looking down the bond between two adjacent atoms. We only draw the front atom. The second atom is obscured behind the first. We also do not show the bond between the two atoms. We do, however, show the other three bonds coming from the two atoms.
Step 1: Find the two bonded atoms which will be at the center of the Newman projection, and draw all the substituents on each atom. (Remember the implicit hydrogens on the carbons.)
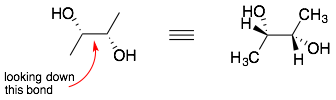
Step 2: Pick a direction to look down the bond (either direction is okay). The carbon closest to you will be the front atom. Draw a circle with three bonds coming out from the center. Attach the three groups to the front atom. If there is stereochemistry then you want make sure that the relative position (left and right) are the same as the stereochemistry of the bond-line drawing.
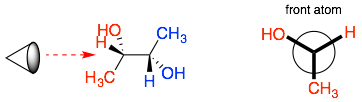
Step 3: Do the same for the back atom. Notice that for the back atom, the bonds to not go the center of the circle because they are behind the first atom.
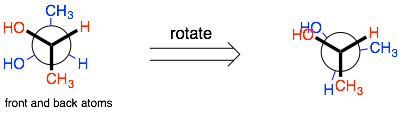
Rotating: To generate different conformers, you can turn either the back or the front (but not both). As you rotate, the molecule should alternate between staggered and eclipsed conformations.