Nucleophile Effects on Substitution Reaction Rates
Introduction:
Nucleophiles have a lone pair that they donate to form a new bond with the electrophile.
For substitution reactions, the nucleophile is a key reactant. Its influence on the rate depends upon the mechanism of the substitution reaction.
SN2 mechanism -> the nucleophile strength strongly influences rate
SN1 mechanism -> the nucleophile strength does NOT influence rate
Therefore, we need to understand nucleophilicity trends (especially for the SN2 reaction.) In general,
MORE STABLE = LESS REACTIVE (ie less nucleophilic)
How to:
In general, the same things that make a molecule a good nucleophile also make it a good base.
First, negatively charged nucleophiles are generally stronger than neutral nucleophiles. For example:
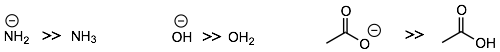
Second, we see the same trends going across a row of the period table (examples (a) and (b)). The more electronegative an atom the less nucleophilic or basic it is because it wants to keep its electrons and not share them.
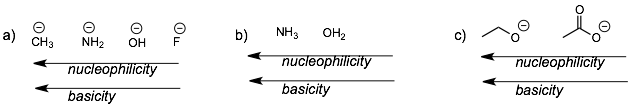
Third, resonance stabilized anions are less basic and less nucleophilic than similar anions without resonance (example (c)).
Some key differences are:
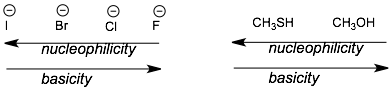
1) Trends going down the periodic table. Nucleophilicity increases as you go down a column in the periodic table but basicity decreases. This is the same trend as we saw above for nucleophilicity, where more electronegative atoms are less nucleophilic because they they hold on to their electrons more tightly.
2) Size trends. Nucleophilicity (especially for SN2 reactions) are very sensitive to the sterics (the size of the nucleophile). In contrast, basicity is not very sensitive to the size of the nucleophile (even a big base can pull off a protons that is sticking off of a molecule). Therefore, with a very strong and big (hindered) base/nucleophile will not be a good nucleophile but still can be a good base.
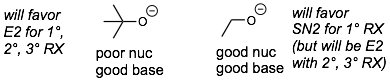
* Note: This discussion of nucleophilicities ignores the possibility of the nucleophile acting as a base. This is particularly problematic for very strong nucleophiles such as NH2- which will usually act as a base and not a nucleophile.