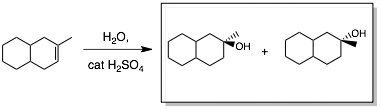
Draw the Products of Alkene Addition Reactions
Introduction:
The next set of reactions that we learn (after the substitution and elimination reactions) are the addition reactions to alkenes. We will also learn 8 to 10 different alkene reactions, which seems a bit overwhelming. However, if you identify the common features of these reactions, then it is pretty easy to learn these reactions as a group.

The individual addition reactions may seem very different, but they all involve the breaking of the C=C pi-bond and the attachment of two new groups (A and B) via single bonds to each carbon of the C=C bond. This could potentially lead to four different products. Each addition reaction will give one or mixture of two of these four products.
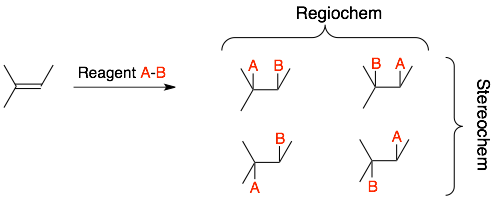
The four products can be grouped into two categories based on:
- Regiochemistry: Is B (the more electronegative negative group) attached to the more or less substituted carbon of the C=C?
- Stereochemistry: Are the A and B groups on the same side (syn-product) or the opposite side (anti-product)?
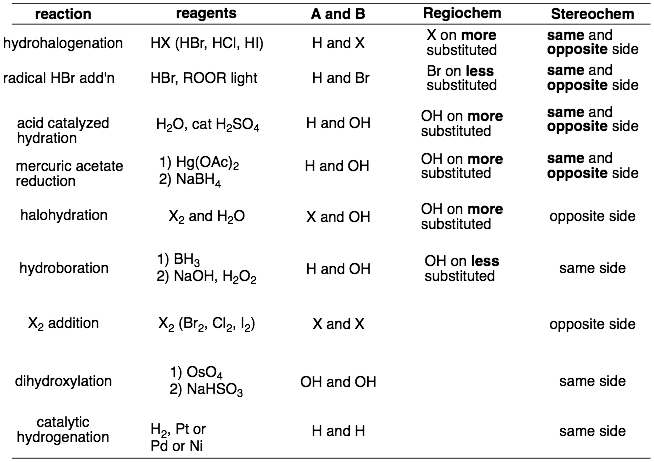
So for each new addition reaction, you need to remember: 1) what are A and B, 2) what is the regiochemistry, and 3) what is the stereochemistry? These are summarized in the table below below.
How to:
Follow the steps in the order below.
Step 1: Identify the alkene addition reaction based the reagents and the A and B groups.
Step 2: Redraw the reactant on the product side and identify the alkene in the reactant by circling it.
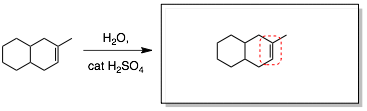
Step 3: Identify the more and less substituted carbon of the alkene and erase the C=C double bond.
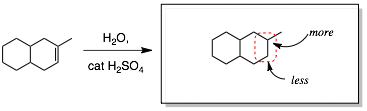
Step 4: Attach the A and B groups, paying attention to attaching A and B with the right regiochemistry.
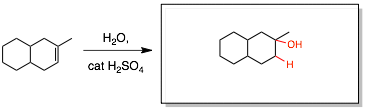
Step 5: Apply the appropriate stereochemistry (whether the A and B groups are on the same or opposite sites or both).

Step 5: Clean up the product by removing any unnecessary hydrogens and checking if the two products are the same.