Draw the Mechanism of Alkene Addition Reactions
Introduction:
The reaction mechanisms of the addition reactions of alkenes have many similarities to the substitution and elimination reactions. The addition reaction mechanisms use different reagents but they all have similar features. 1) They the C=C pi-bond will break (by swinging out) to form a single bond to A and 2) the other carbon of the C=C bond will form a bond to B. The main difference is the order in which these two steps occur and what A and B are.

How to:
1) There are two general alkene addition mechanisms. These are the two-step and one-step mechanisms. (They are analogous to the two-step and one-step substitution mechanisms).
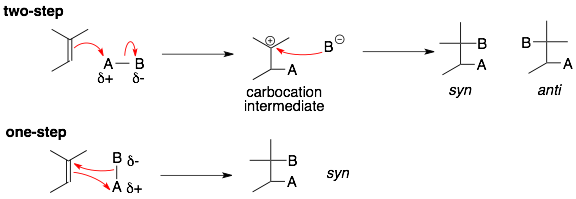
a) In the two-step mechanism, the A- and B-groups are attached sequentially, one at a time. First, the C=C bond swings out to form a bond with the A-group. This forms the key carbocation intermediate. Second, the B-group forms a bond with the carbocation carbon. Reactions with two-step addition mechanism include:
HX addition (HCl or HBr)
hydration (H3O+ or H2SO4, H2O)
bromination (Br2)
hydrobromination (Br2, H2O)
oxymercuration (HgOAc2, H2O)
b) In the one-step mechanism, the A- and B-groups are attached at the same time. There is no carbocation intermediate. Reactions with concerted, one-step mechanisms include:
hydroboration-oxidation (1. BH3, 2. NaOH, HOOH)
dihydroxylation-oxidation (1. OsO4, 2. NaHSO3)
oxanolysis (1. O3, 2. Me2S)
hydrobromination (Br2, H2O)
hydrogenation (H2, Pt or Pd or Ni)
2) Three-membered ring intermediates: One variant of the two-step addition reaction mechanism is the formation a three-membered ring (positively charged) intermediate.

The first step is identical. The C=C bond swings out to form a bond with the A-group. This forms a carbocation. However, this carbocation is able to form a resonance structure that is a 3-membered ring. This three-membered ring intermediate is the major resonance structure and is a better representation of the cationic intermediate than the carbocation. Examples of 3-membered ring intermediates are shown below.
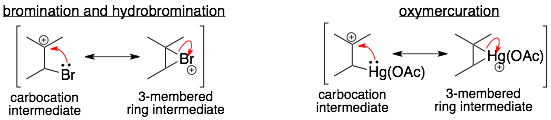
One key difference between two-step addition reactions that can and cannot form the 3-membered intermediate is the stereochemistry of the final product. In reactions that form the 3-membered ring intermediate, the A- and B-groups are on opposite sides of the product (anti). In contrast, in reactions that form the carbocation intermediate and not the three-membered intermediate, both the syn– and anti-products are formed.