Acidity Trends
Introduction
Estimating pKa’s is one of the most useful in predicting reactivity trends in organic chemistry. The most accurate methods are using the pKa table or making estimates based on the functional groups. However, in some cases, we need to differentiate between molecules with the same functional group, or the functional group is not in our pKa table. In these cases, we need to estimate the pKa using the acidity trends. These acidity trends can also be useful in explaining observed acidity trends.
(WARNING) These acidity trends should only be used if the pKa or acidity trends cannot be estimated using the pKa table or functional group table. The reason is that the trends often conflict (in other words they would give different answers) and in these cases, it is not clear which trend takes priority.
How to:
There are no hard and fast rules for which acidity trends take priority. This is why these trends should only be used as a last resort. However, trends (1-3) where the differences are in the atom directly attached to the acidic hydrogen usually have larger effects than trends (4-6) where the differences are further from the acidic hydrogen.
(Trend 1) When comparing acids that have the same element attached to the acidic proton, acids where the attached atom is positively charged will be more acidic than acids with a neutral attached atom.
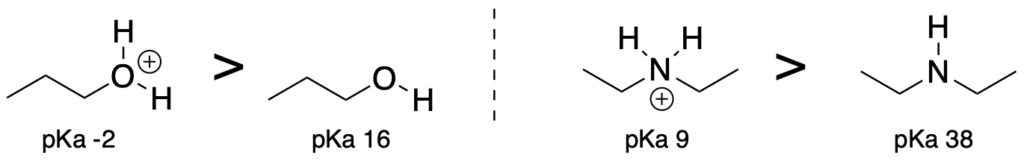
(Trend 2) Acids with the more electronegative atom attached to the acidic proton will be more acidic. (Use when comparing elements which are in the same column of the periodic table.)
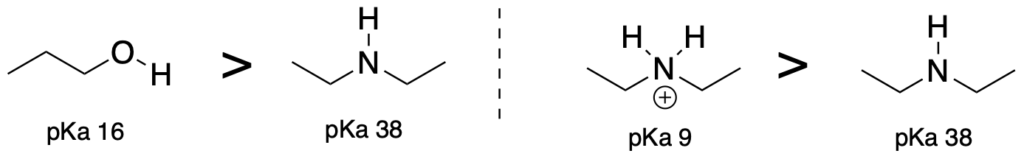
(Trend 3) Acids where the atom attached to the acidic proton are larger will be more acidic. (Use when comparing elements which are in the same row of the periodic table)
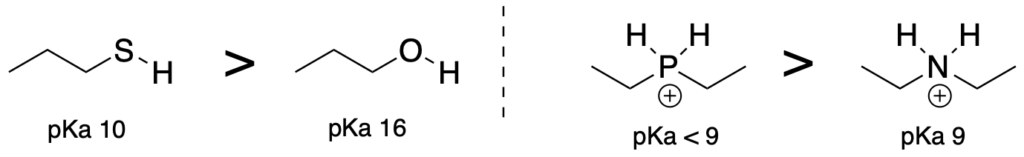
(Trend 4) Acids where the conjugate base is stabilized by resonance are more stable than acids where the conjugate base is not stabilized by resonance. (The atoms attached to the acidic proton should be the same.)
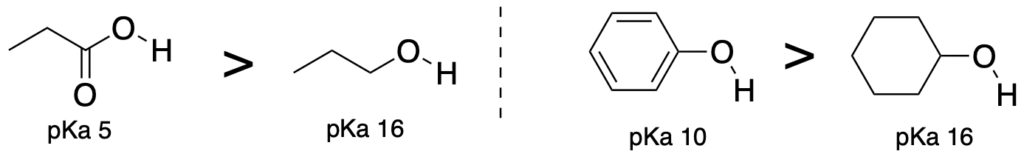
(Trend 5) Acids where the atom attached to the acidic proton has more S character is more acidic. Therefore, SP > SP2 > SP3. (The atoms attached to the acidic proton should be the same.)
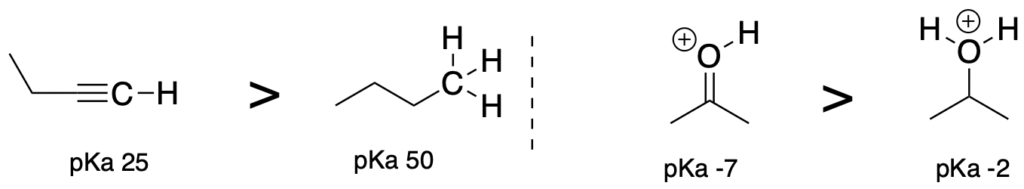
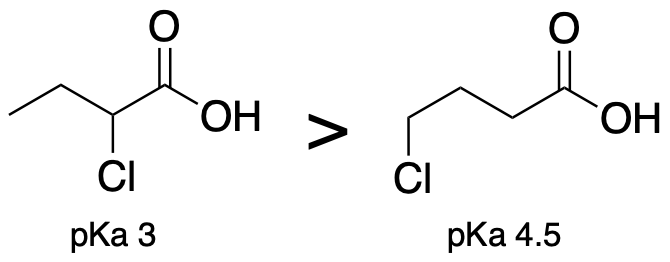
(Trend 6) Acids where electronegative groups are fewer bonds away from the acidic proton are more acidic. (The atoms attached to the acidic proton should be the same.)