Is a bond ionic or covalent?
Introduction:
An ionic bond is where one atom takes one valence electron from the other. This will give one atom a positive formal charge and the other a negative formal charge. The two atoms stay together because of the electrostatic attraction of the plus and minus charges.
A covalent bond is where two atoms share two electrons. They do this to try to fill their valence shells. Covalent bonds have well defined lengths (~1.0 to 2.5 Å) and bond strengths (~100 kcal/mol).
How to:
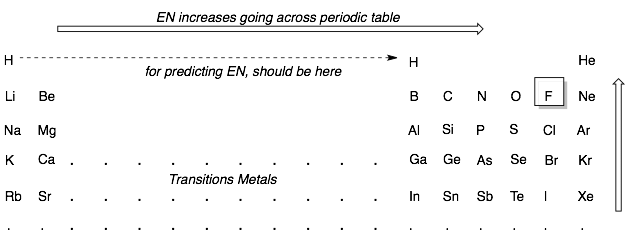
One way to predict whether a bond is ionic or covalent is to look how far apart the two atoms forming the bonds are in the periodic table. If one atom is on the far left (Group 1 or 2) and the other is on the far right (Group 5, 6, or 7), then the atoms will have large differences in EN and will form an ionic bond. Most other pairs of atoms are close enough in EN to form covalent bonds. For example, any two atoms in the main group elements (Groups 3, 4, 5, 6, 7), will usually form a covalent bond. Remember to treat hydrogen like a Group 3 element as its EN is closest to boron.
Examples:
Na and Cl –> ionic
K and O –> ionic
Ca and O –> ionic
C and F –> covalent
Si and Cl –> covalent
C and C –> covalent
C and H –> covalent
B and F –> covalent
Advanced:
Covalent and ionic are just the two extremes of the different types of bonds. There is actually a continuum of bond types. Most bonds have some covalent and some ionic character. For example, a C-C covalent bond has little or no ionic character because the two atoms have the same EN’s. The Si-F is one of the most polarized covalent bonds and has a lot of ionic character. The N-Li bond is ionic but has significant covalent character.