Recognizing invalid or unlikely Lewis Structures
Introduction:
In teaching, we usually only show examples of correct structures. However, it is useful to be able to recognize invalid or unlikely Lewis Structures. This is particularly helpful in quickly narrowing down the answers.
How to:
Try to follow the following rules when drawing Lewis Structures:
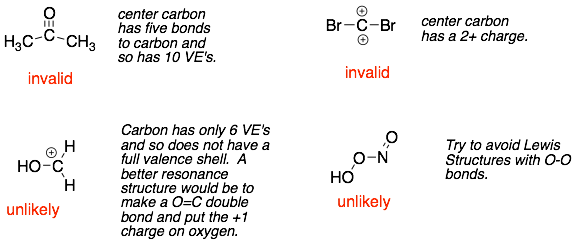
NEVER: exceed the octet rule for second row atoms (B, C, N, O, F). This is easy to see now. But later we will stop showing lone pairs and hydrogens attached to carbons, which will make it harder to identify atoms breaking the octet rule.
NEVER: have a formal charge greater than +1 or less than -1 for main group atoms (Groups 3-7). For example, carbon, nitrogen, or oxygen should never have a 2+ or 2- charge.
AVOID: drawing Lewis structures with O-O single bonds because these structures are very unstable. The O-O single bond is very weak (about 1/3 of a C-C) and will break very readily. We will use this later in the class to make very reactive reagents.
AVOID: drawing Lewis structures where one or more atoms does not have a full valence shell. Again, these structures will be very reactive (i.e. unstable), and they do exist. However, there is probably a better Lewis structure where all the atoms have a full valence shell.
Note: The number of bonds for charged atoms is always different (with one extra or one less bond) than for neutral atoms. For example, a neutral carbon has four bonds. A positively charge carbon has only three bonds and a negatively charged carbon also has three bonds (and one lone pair).
Examples: