Approach to Synthesis Problems
Introduction:
Synthesis problems are a new type of problem. They involve identifying a sequence of two or more reactions that will transform the molecule on the left to the final product on the right. You see begin to see these types of problems toward the end of the first semester of organic chemistry, once you have learned a larger enough pool of reactions. However, you will see a lot more of this type of problem throughout the entire second semester of organic chemistry.
How to:
The best strategy is to simultaneously work forward from the molecule on the left (forward synthesis) and work backwards from the molecule on the right (retrosynthesis). List all the possible reactions you can think about that involve the molecule on the left as a reactant (blue arrows). At the same time, think about all of the reactions that would give the molecule on the right as the product (red arrows). Keep working forward and backwards and hopefully, the two will converge. You will see the same structure left as on the right.
Once you have picked a viable route, you need to fill in all the reagents for each step.
Two examples are shown below. These synthesis problems require knowledge of the following reactions: radical bromination of alkanes, addition reactions of alkenes and alkynes, substitution reactions (SN1 and SN2), elimination reactions (E1 and E2)
Additional advice on how to approach and executing strategies to answer synthesis problems are at the bottom of the page after the two examples.
Example 1:
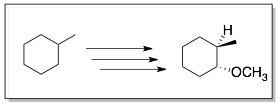
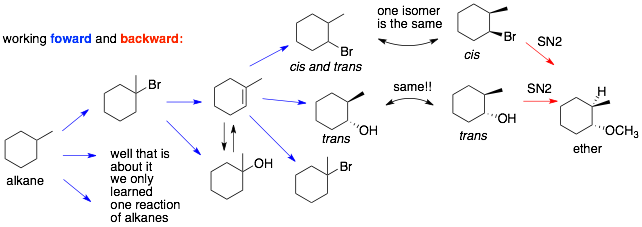
Comments: Two feasible synthetic routes were identified. The top route 1 actually forms a mixture of cis- and trans-products that need to be separated halfway through the synthesis. The bottom route 2 gives the correct trans-product and thus, no separation of products is required.
Filling in the reagents for the two route gives the final answers. Note, that on an exam you need only show one viable synthetic route (with reagents). I have just shown two separate routes to give you more examples and practice.

Analysis of Route 1: First, radical bromination gives the more substituted alkyl bromide. E2 elimination with a strong base (NaOEt) gives the more substituted alkene. Anti-Markovnikov HBr addition (under radical conditions) puts the Br on the less-substituted carbon of the C=C bond. However, we run into our first problem. This reaction is not stereoselective and gives a mixture of cis and trans products. We only want the trans product. Using only the trans product, we try to carry out an SN2 reaction with CH3O–, which should invert the stereocenter. However, we run into our second problem and this one is fatal. To get SN2 we need a good nucleophile like CH3O– (if we use a weak nucleophile like CH3OH, we get SN1/E1). However, using a strong base/nuc with a 2° alkyl halide does not go SN2. It will go E2.
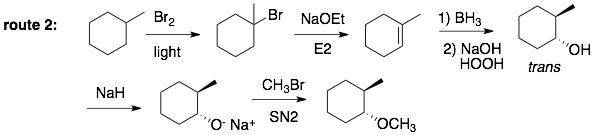
Analysis of Route 2: The first two steps are the same as route 1. The hydroboration reaction, however, gives only the isomer that we want. Deprotonation of the alcohol with a strong base will make it a good nucleophile, which we can use in the Williamson ether synthesis (SN2). Reaction with CH3Br (which cannot eliminate) will go SN2 to give the desired product. Also the stereochemistry does not change because the alkoxide is now the nucleophile.
Example 2:
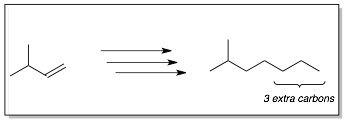
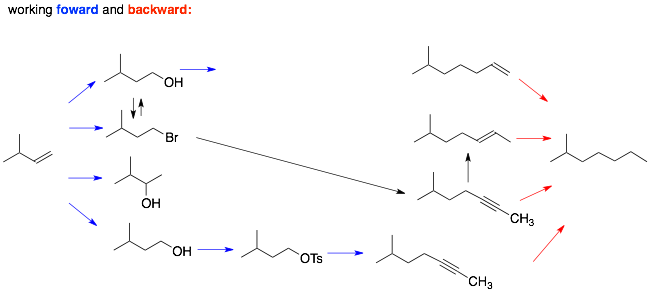
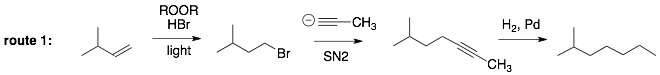
Analysis of Route 1: The only way we learned to add more carbons to our molecules is using the anion of an alkyne as a nucleophile (SN2). The alkyne can then turned into an alkane (via H2 Pd) or into an alkene (Lindar’s catalyst or Na, NH3). In order to do this in this synthesis, we need to put a leaving group onto the end of the molecule on the left using an anti-Markovnikov addition reaction to the alkene.
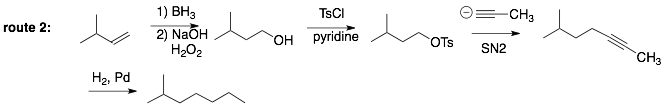
Analysis of Route 2: A similar approach is used where a three carbon chain is introduced via the anion of propyne. In this case, the alkyne nucleophile is added to the epoxide. Note, that the nucleophile adds preferentially to the less substituted (i.e. crowded) carbon of the epoxide. However, this still leave an alcohol on the other carbon which we can remove by elimination to the alkene, which is transformed into an alkane along with the alkyne using H2, Pd.
General Advice:
1) You need to memorize and be able to quickly recall all (or at least a reasonable subset) of the reactions that we have covered. The best way is to try to organize the reactions. One way is pictorially, using the handout of the ‘map’ of the reactions that shows how they are interconnected. The second way is by categories, using the several page handout that lists the reactions in groups by the functional groups.
2) There are many possible routes to the product, which is initially a bit confusing but it can also help you out. For example, if you have forgotten one of the key reactions, you can often figure out a synthetic route that works around the missing reaction.
3) If you can make an alkene, alkyne, or alcohols, this really opens up the number of options because we learned a lot of reactions of these functional groups. Remember in the ‘map’ of reactions, the alkene and epoxide molecules were in the center of the ‘reaction webs’.
4) Even if you cannot find a continuous series of reactions, try to show as much as possible (including reagents) because you can get partial credit.
5) Remember that the order that you do the reactions can lead to very different products.
6) When doing a reaction you must make sure that the product you want will be a major product. For example, if you are doing an elimination you will typically form the more substituted alkene. Alternatively, if you are doing a radical bromination, the major product will be the most substituted alkyl halide