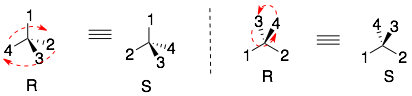Assign Chiral Centers R or S
Introduction:
In the IUPAC naming system, each chiral center is assigned as either R or S. Remember that chiral centers (also known as stereogenic centers) are tetrahedral atoms with four different attached groups. Usually, chiral centers are SP3 hybridized carbons.* The four groups can be attached in two different ways, leading to enantiomeric forms (R and S).
How to:
There are two steps to assigning a chiral center.
STEP 1: The four different groups attached to the chiral center atom are ranked from highest priority (1) to lowest priority (4).
STEP 2: The chiral center is reoriented so that the lowest priority group is placed in the back and the remaining groups are connected in order of priority. If these groups (1, 2, and 3) are in a clockwise order then the chiral center is R. If the groups (1, 2, and 3) are in a counterclockwise order then the chiral center is S.
Rules for each of these steps are described in more detail below.
(STEP 1) Rules for ranking the priority of groups attached to the chiral center
1) Start with the first atom of each group that is directly attached to the chiral tetrahedral center. The atoms with higher atomic numbers will have the highest priority. For example, the ranking for the four groups around the chiral center of the molecule CHBrClF would be:
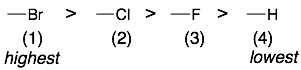
Hint: If hydrogen is one of the groups attached to a chiral center, then it will be the lowest priority group.
2) If two groups have the same first atom, then compare the second atom from the chiral center. If there are multiple second atoms, then compare them in order of atomic number. Stop, when there is a difference. For example:

HINT: It is important to stop when you find a difference. Don’t get fooled by seeing higher atomic number atoms on the substituent that are further from the chiral center, like in the top example.
3) If all the first and second atoms from the chiral center are the same, then proceed to the next furthest atom (in order of atomic number) until you find a difference.
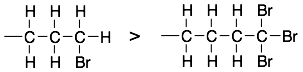
4) Treat double and triple bonds as if they are a series of single bonds to the same atom. This will involve the creation of singly bonded dummy atoms (highlighted in blue) of the same type as involved in the double or triple bonds. For example:
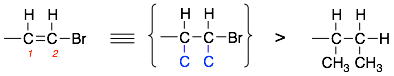
Note: The first carbon is singly bonded to hydrogen and doubly bonded to carbon 2. For the purposes of ranking priorities, we would consider the first carbon as being singly bonded to hydrogen, singly bonded to carbon 2, and singly bonded to another ‘dummy’ carbon.
The same rules can be applied to carbon two, it is singly bonded to a H and Br and doubly bonded to carbon one. Therefore, we would consider carbon two as being singly bonded to H, Br, and carbon one. In addition, it also forms a single bond to another ‘dummy’ carbon.
(STEP 2) Rules for assigning R and S.
1) After assigning priorities to the four different groups attached to the chiral center, make sure that the lowest priority group (4) is pointing away from you. All three examples below have the lowest priority (4) group point to the back.
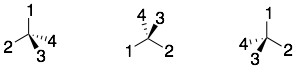
2) Connect the groups in the order: 1, 2, and 3. If the direction is clockwise, then the chiral center is R. If the direction is counter-clockwise, then the chiral center is S.

Hint: One trick that I use is to use my right hand. I point my thumb in the direction of the lowest priority group (4), and then curl my fingers (like in a grabbing motion) in the direction of groups 1, 2, and 3. If my fingers follow the direction of 1, 2, and 3, then the chiral center is R. This is easy to remember (right = R). If my right-hand does not follow the direction of 1, 2, and 3, then the chiral center is S.

Method B: Switch any two groups, which will flip the chiral center. This method is easier to implement but it is a bit confusing because it will invert the chiral center. So, if you use this method for assigning the chiral center, you need to switch your final assignment.