Calculating the formal charge on an atom
Introduction:
Sometimes atoms will have extra or not enough electron electrons. This imbalance of electrons is denoted by a formal charge. A negative formal charge means there are too many electrons on an atom. (Remember electrons are negatively charged.) A positive formal charge means there are not enough electrons on an atom. One confusing thing about formal charges is that we do not simply count up all of the electrons around an atom. Different types of electrons are counted differently. Non-bonding electrons are counted individually. However, electrons in bonds are counted as being shared and so each pair of electrons in a bond counts as only one electron.
How to:
To calculate the formal charge of an atom, use the equation below. Take the valence number of the atom and subtract the number of bonds and the number of non-bonding electrons.
Formal Charge = (valence number) – (number of bonds) – (non-bonding electrons)
Examples of the calculation of the formal charge for bonded atoms.
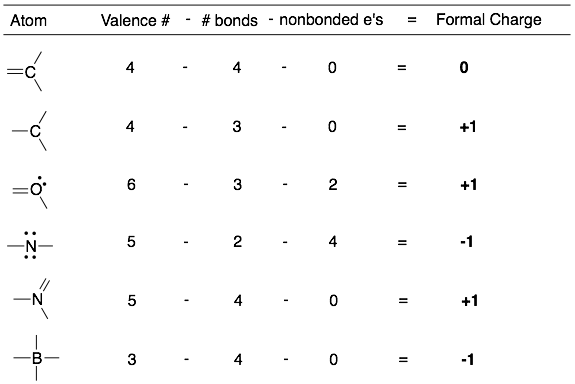
Comments:
Counting electrons to calculate formal charges is different from counting electrons for octets. Shared electrons (in bonds) and unshared electrons (in lone pairs) are counted differently.
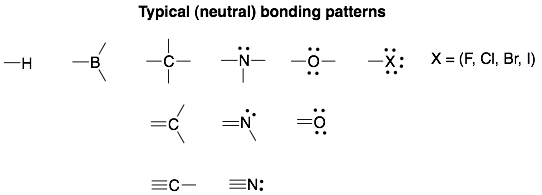
Hint: Instead of calculating the formal charge for every atom in a molecule, it is a lot faster to identify the atoms that deviate from the “typical” bonding patterns. The typical bonding patterns will be neutral (formal charge = 0). So atoms that deviate from these will usually have a formal charge.
Examples:
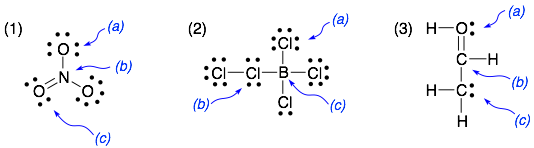
Answers: 1a = -1, 1b = +1, 1c = 0, 2a = 0, 2b = +1, 2c = -1, 3a = +1, 3b = 0, 3c = -1