Classification of Solvents
Introduction:
There are two general categories of solvents. These are:
- polar/non-polar
- protic/aprotic.
These categories are important because reactions can prefer (go faster) one class of solvent over another.
For example, reactions that have a charged or partially charged transition state or intermediate will go faster in polar solvents. This turns out to be most reactions including SN1, SN2, E1, and E2 reactions.*
Similarly, reactions that form anions in the transition state or intermediates will tend to go faster in protic solvents like SN1 and E1 reactions. Alternatively, reactions that have anionic reactants will tend to go slower or not at all in protic solvents (SN2 and E2). In both cases, the reason is that protic solvents can hydrogen bond to and stabilize anionic species.
How to:
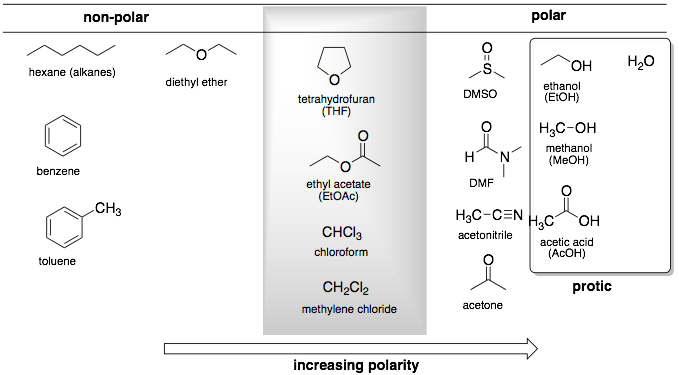
Polar solvents are ones that have a strong dipole moment. Therefore, polar solvent are good at stabilizing polar or charged transition states and intermediates. Usually, polar solvents will have one or more electronegative atoms like O or N (see table below). Common functional groups in polar solvents include alcohols, carboxylic acids, carboxylic amides, and ketones.
Protic solvents have an acidic hydrogen (pKa < 18). Common functional groups are alcohols, carboxylic acids, and water. Note that all protic solvents are polar solvents. Solvents that are not protic solvents (pKa > 18) are called aprotic solvents.
Advanced Topics
While most reaction rates will be go faster in more polar solvents, the exceptions are reactions that proceed via neutral radical intermediates or reactions with concerted cyclic neutral transition states (i.e. cycloadditions, ene-rearrangements etc….)