Determine the Isomeric Relationship Between Two Molecules
Introduction:
Chemists like to categorize the similarities between two molecules just as you would for the relationship between two people. The level of similarity between two molecules can help predict their similarity in properties and chemical reactivity.
Two molecules that are very similar are called isomers. The problem is that there are many different kinds of isomers. These are listed below in order of increasing similarity. Note that there are two kinds of stereoisomers: diastereomers and enantiomers.
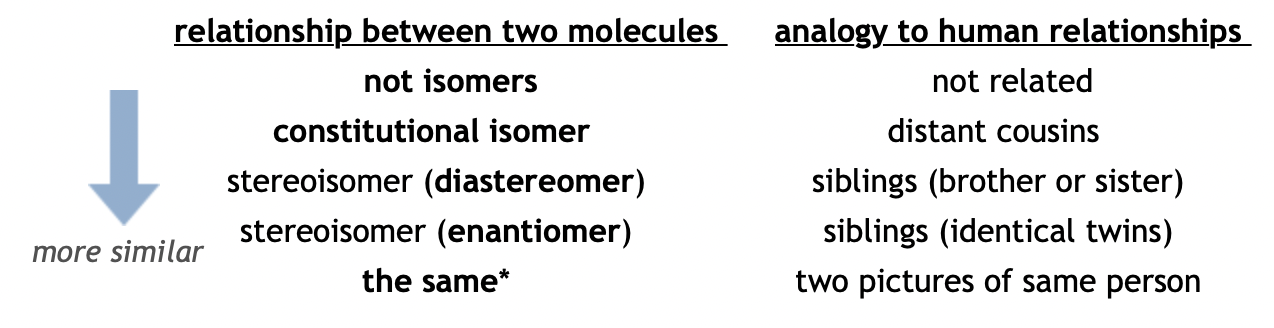
*Conformational isomers or rotamers are the same molecules that have been folded or twisted into different three dimensional structures. A human analogy would be two pictures of the same person with their hands up and hands down.
How to:
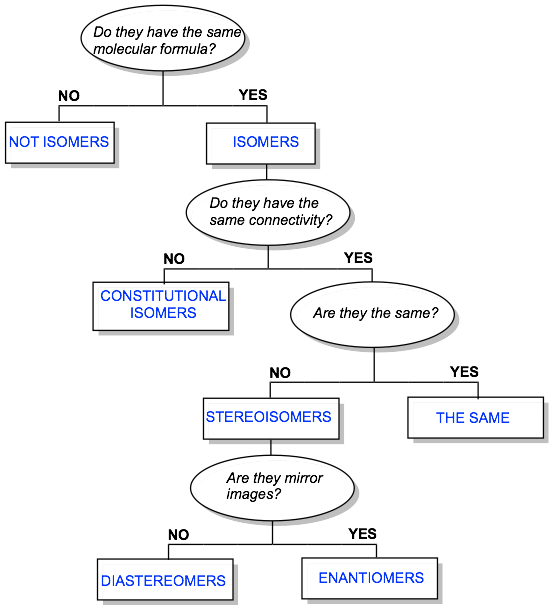
Below is a flow chart to help you categorize the relationship between two molecules. The possible answers are: a) not isomers, b) two different depictions of the same molecule, c) constitutional isomers, d) diastereomers, and e) enantiomers.
Example 1
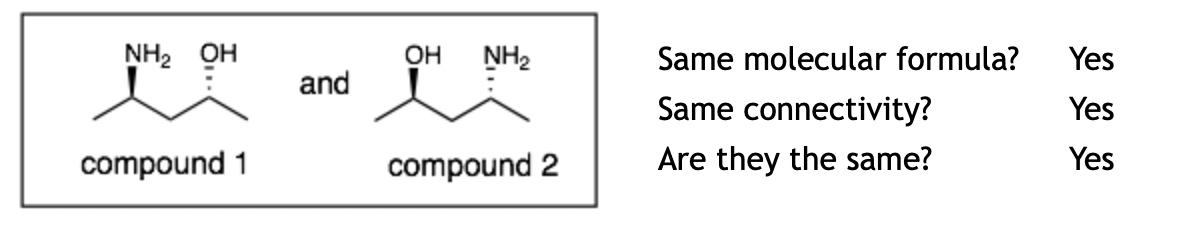
Explanation: These two are the same molecules. If you pick up compound 1 and flip it over (around the horizontal-axis), you get compound 2. Notice that when you flip over the molecule the groups pointing up will point down and visa versa.

Example 2:
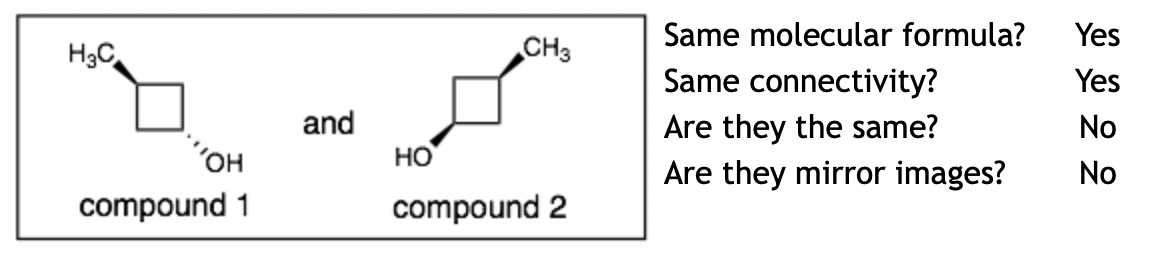
Explanation: These molecules are diastereomers. They are not mirror images. We can try to test this by drawing the mirror image of compound 1 and checking to see if it is the same as compound 2 (which it is not).

Example 3:
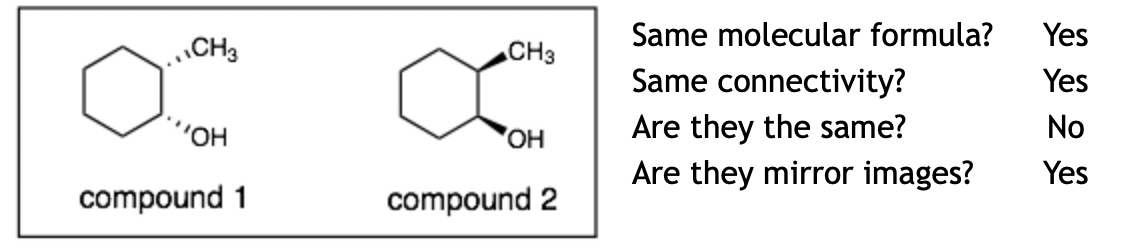
Explanation: These molecules are enantiomers. The hard question is whether these molecules are the same. It looks like you could flip over compound 1 and it would become compound 2. However, if you did that, then the CH3 and the OH groups would switch places and be at the wrong positions. We can also tell these are mirror images if we place the mirror behind compound 1. This would flip all of the chiral centers (without moving their positions) and this mirror image of compound 1 is the same as compound 2. Therefore, 1 and 2 are mirror images of each other and are different, which is the definition of enantiomers.
Example 4:
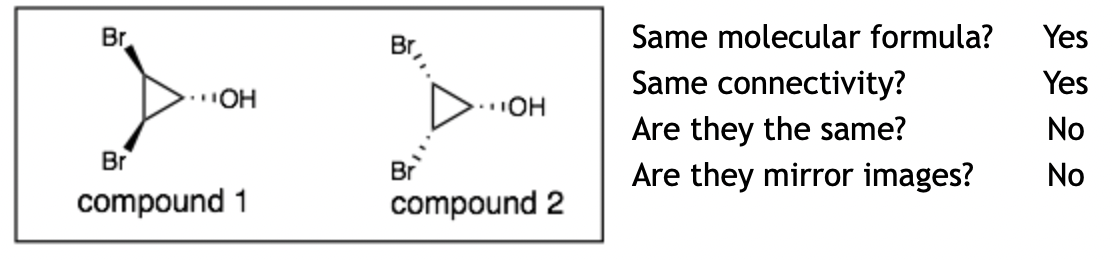
Explanation: These molecules are diastereomers. The hard question is: “are they the same?” If you flip over compound 1, then the Br’s that point up will point down just like in compound 2. However, remember that the OH will also change stereochemistry from down to up, which is different from compound 2.

Example 5:
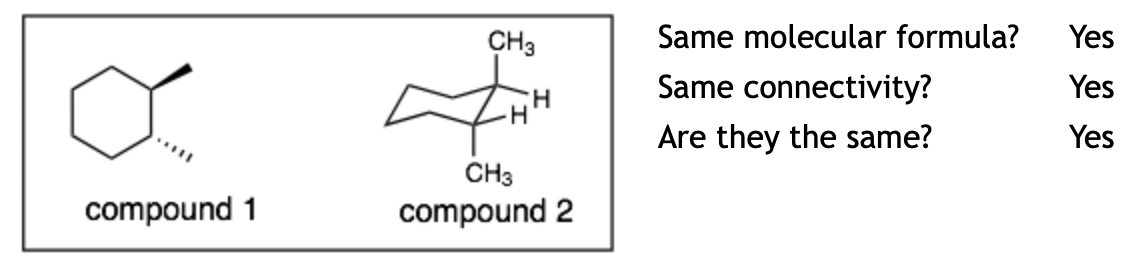
Explanation: The molecules are the same. This problem is difficult because the two cyclohexanes (compound 1 and 2) are depicted from different perspectives. In compound 1, we are looking from the top of the cyclohexane and in compound 2 we are looking from the side the cyclohexane.) The hard question is again: “are they the same.” The difficulty is in deciding in compound 2 if the methyl groups are both up, both down, or one up and one down. Remember that each carbon in a ring will have two substituents (one up and one down). We can see that in compound 2, one methyl group is up and the other is down.
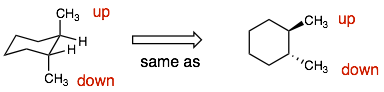
Example 6:
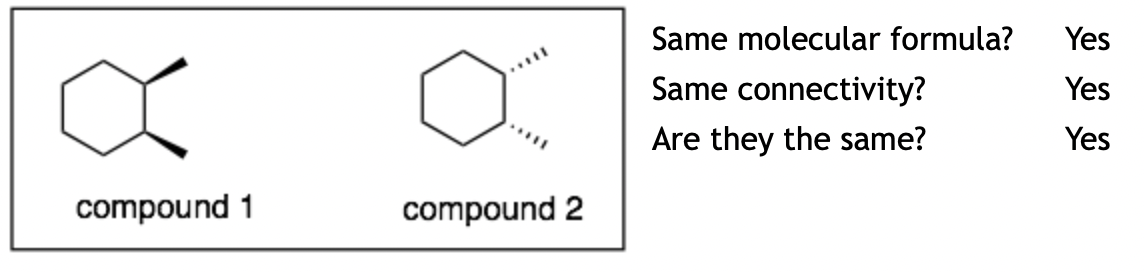
Explanation: The molecules are the same. Again the difficult question is: “Are they the same?” If you flip over compound 1, then it will be the same as compound 2.
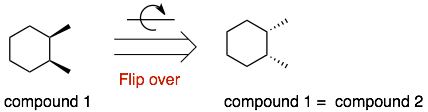
Example 7:
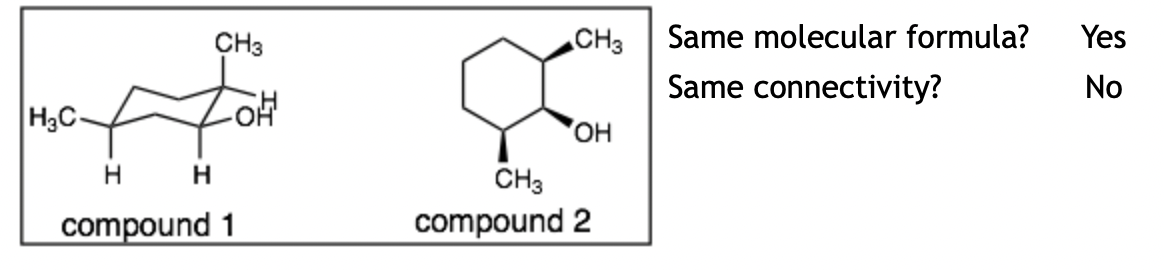
Explanation: The molecules are constitutional isomers. Like example 5, the problem is translating the two different perspectives of the cyclohexane ring. It is helpful to redraw the chair depiction (compound 1) in the top-view depiction (like compound 2). When we do this, we can clearly see that the connectivity of these isomers is different and therefore, these compounds are constitutional isomers. (Hint: it is helpful to number the carbons when doing this translation.)
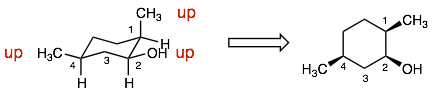
Note: It is hard to tell if the OH and CH3 groups on carbons 2 and 4 are up or down. However, it is easier to see that the H’s on these carbons are both down. Therefore, we know that the other substituent (the OH and CH3) must be up.
Example 8:
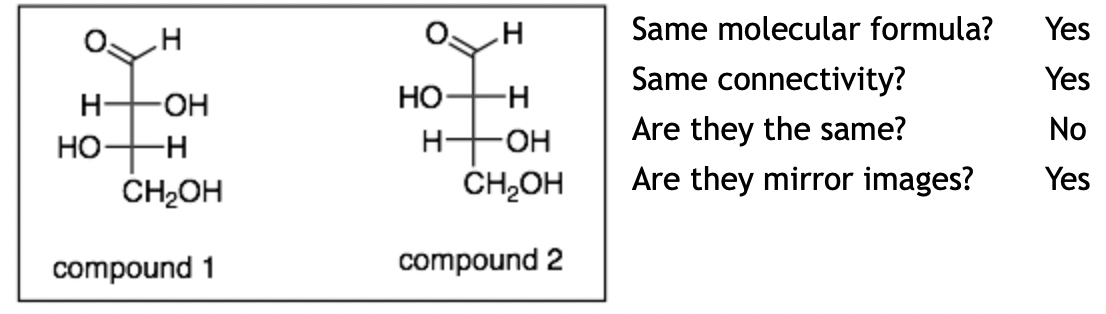
Explanation: The molecules are enantiomers. These molecules are shown as Fischer projections. The nice thing about Fischer projections is that it makes comparing stereochemistry easier. You can just compare chirality centers simply by looking at which groups are on the left and right sides. For example in compound 1, the top chirality center has the OH group on the right; whereas, in compound 2, the top chirality center has the OH on the left. So, the top chiral centers of the two molecules are opposites, as are the bottom chirality centers. Therefore, these molecules are enantiomers.