Draw the Products of Elimination Reactions
Introduction:
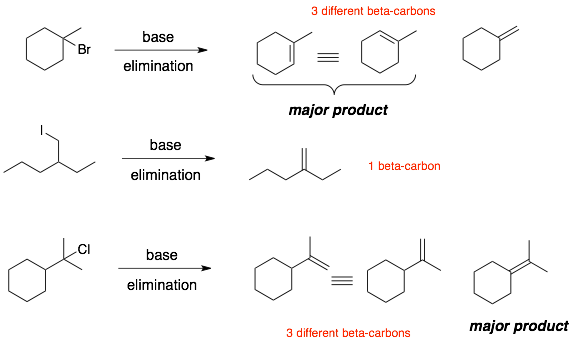
The products of elimination reactions are more difficult to draw than simple substitution reaction. The reason is that the reaction occurs not only at the alpha-carbon (where the LG is attached) but also at the adjacent beta-carbon.
How to:
Step 1: Identify the alpha-carbon, which is the carbon where the LG is attached.

Step 2: Identify the beta-carbon (an adjacent carbon) and make sure that it is: 1) SP3 hybridized and 2) that it has at least one hydrogen.

Step 3: Remove (delete) the LG and make a double bond between the alpha- and beta-carbons. (If there is more than one beta-carbon, then there will be multiple products.)
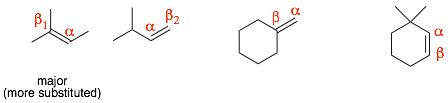
Examples:
Advanced Topics (Stereochemistry):
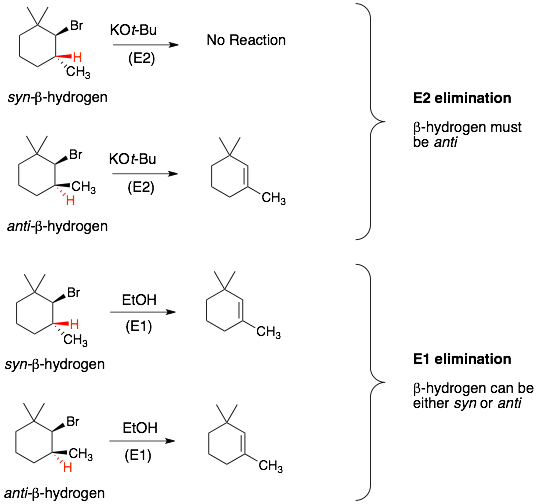
1) cis and trans alkenes: In most cases, if you the alkene product could have cisand trans isomers, then both isomers will be formed. The more stable trans isomer will be the major isomer
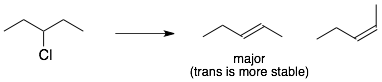
2) syn– and anti-beta hydrogens: For E2 eliminations, the hydrogen on the beta-carbon must be anti– (or on the opposite side) to the LG. For E1 eliminations, the hydrogen on the beta-carbon does not have to be anti.