Drawing Lewis Structures from an molecular formula (part 1)
Introduction:
By counting valence electrons, we can predict the stable molecules that could form for a particular molecular formula. Molecular structures that are drawn using this formalism (of counting valence electrons) are called Lewis Structures. In a Lewis Structure, each pair of shared electrons in a bond are denoted by a line between the two atoms. The unshared electrons may or may not be shown. However, the formal charges on atoms should always be shown.
How to:
There are many strategies for generating Lewis Structures from a molecular formula. Most books first calculate the total number of electrons in a molecule and then distribute them among the atoms. One shortcut is to start with the typical bonding patterns for each atom in the formula. Then try to connect the atoms together so that there are no “dangling” bonds (i.e. bonds with only one atom). See the Handout on Identifying Invalid or unlikely Lewis Structures to help eliminate the number of possibilities.
Example 1: The molecular formula is on the left and the Lewis Structure is in the box on the lower right.
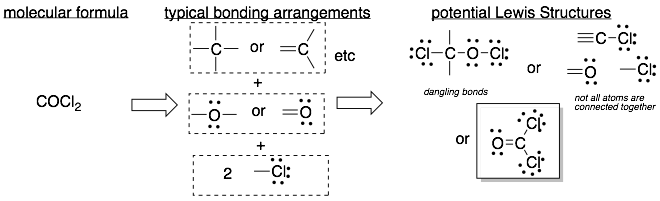
Hint: Start building Lewis Structures with only single bonds. If there are dangling bonds, then you should try structures with double and triple bonds (or rings).
Example 2: The molecular formula is on the left and the Lewis Structures are in the boxes on the right.
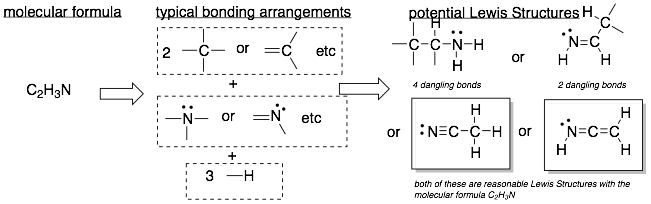
Hint: There are often multiple valid Lewis Structures (LS) for a given molecular formula. However, you should try to stick to LS where all atoms fill their valence shells because these are more stable molecules. Also, avoid try to avoid LS with O-O or O-X(halogen) bonds because they are very weak bonds.
Note: For this exercise, the lone pairs in the Lewis Structures are shown for clarity. However, most Lewis Structures are shown without the lone pairs which are implicit (which means you have to know that they are there even though they are not shown).