Drawing Lewis Structures from a molecular formula (part 2)
Introduction:
In some cases, atoms in a Lewis Structure will deviate from the typical neutral bonding patterns. This will happen if the molecule has an overall charge (+1, -1, +2, -2, etc.) but it can also occur in neutral molecules.
How to:
These types of problems are much more difficult. Do the problem as before and try to attach all the atom fragments together without any dangling bonds. Most of the atoms will adhere to the typical bonding patterns. Usually, one or two atoms will deviate from the typical patterns and instead will follow one of the bonding patterns below. Unfortunately, this will increase the number of possible combinations. Luckily, we can restrict ourselves to atoms with +1 and -1 formal charges because atoms with +2 and -2 charges do not commonly occur in organic molecules.
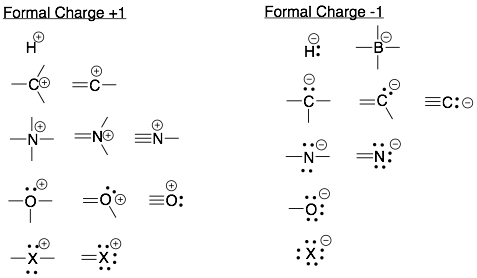
Note: The number of bonds for charged atoms is always different (with one extra or one less bond) than for neutral atoms. For example, a neutral carbon has four bonds. A positively charged carbon has only three bonds and a negatively charged carbon also has three bonds (and one lone pair).
Examples:
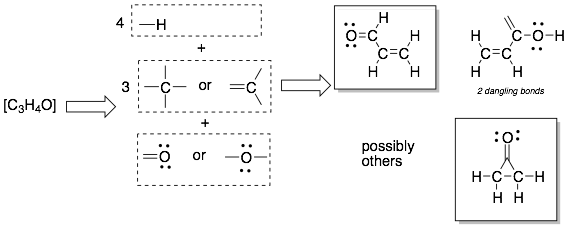
The molecular formula is on the left and the Lewis Structure is in the box on the right.
Example 1
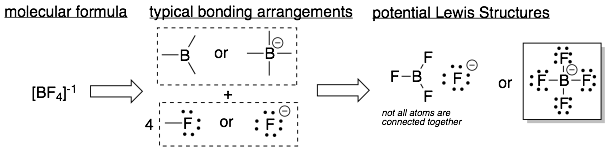
Example 2:
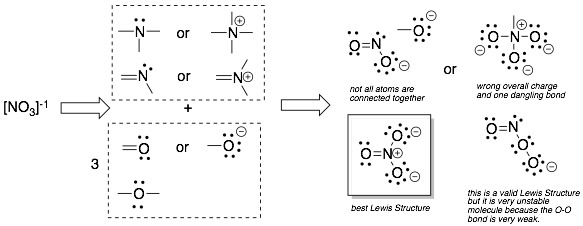
Example 3:
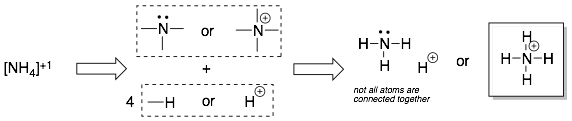
Example 4:
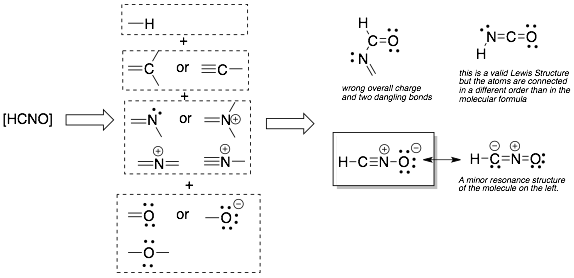
Note: Even in neutral molecules, sometimes individual atoms can have a formal charge. Luckily, carbon will rarely have a formal charge in a neutral molecule. Nitrogen and oxygen often have formal charges in neutral molecules.
Example 5: