Elimination Reaction Overview and Traits
Introduction:
The elimination reactions of alkyl halides (R-X) is taught along side the substitution reaction. The reaction involves the loss of the leaving group (X) and a hydrogen (H) and the formation of a carbon-carbon double bond (C=C).
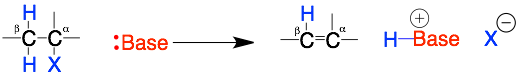
This reaction is confusing for two reasons: 1) the reactants are the same as the substitution reactions (Nuc/base + R-X) but they give different products and 2) the reaction involves a more complex bond making and breaking mechanism that involves two adjacent carbons (alpha and beta).
Mechanisms:
There are two general substitution reactions (shown below) are E2 and E1. Pay close attention to the number of steps and order of steps in a mechanism. Think about which bonds get formed and broken in each step.
E2 Mechanism
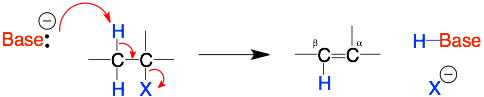
Comments: The key step is the base grabbing the proton on the beta carbon. Therefore, 1) a strong base is required, 2) the proton is sticking out and so even a bulky base is okay, and 3) the beta-proton must be anti– (on the opposite side) to the leaving group (X).
E1 Mechanism
E1: This is a two-step mechanism. The R-X bond first breaks. This step is slow. Then the nucleophile forms a bond to the carbocation intermediate (R+).
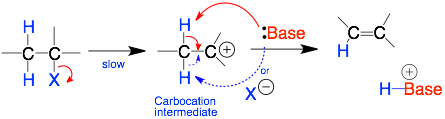
Comments: The key step is the formation of the positivelycharged R+ intermediate. So, R will be 3° or 2° to form more stable carbocation intermediates. The carbocation intermediate is flat. So, the base can grab either beta-carbon proton. The base is usually weak (and neutral and protic) because it is not involved in the key step.
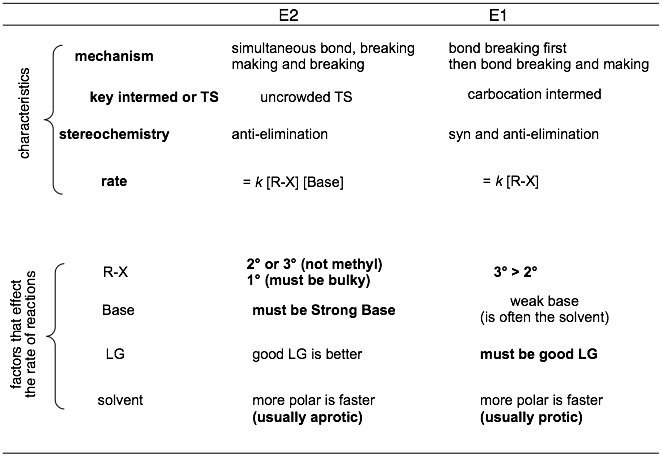
Summary of key traits: