Find the most basic atom in a molecule
Introduction:
Bases are molecules that can accept a proton (H+). Basic atoms in a molecule will have lone pairs, to accept the proton. One problem is that the lone pairs are not always shown,. The lone pairs can be on a neutral (uncharged) atoms (N, P, O, S) or on an anionic (negatively charged) atoms (C, N, O, S, X).
Molecules can also contain multiple basic atoms. However, only the most basic lone pair will usually participate in an acid-base reaction. Therefore, it is important to be able to identify the most basic lone pair in a molecule.
How to:
Here are two methods for finding the most basic lone pair. These methods should work when comparing the basicity of two different molecules or two different lone pairs in a single molecule.
- (Most direct method) Compare the stability of the two lone pairs in question. The more stable lone pair will be the weaker base. For example, a lone pair on a neutral atom will be more stable and less basic than a lone pair on a negatively charged atom. For lone pairs on atoms of the same charge, the lone pair on the more electronegative atom or with more resonance structures will usually be more stable and less basic.
- (Indirect method) Convert the bases into their corresponding conjugate acids. Then compare the acidty (pKa’s) of the acids. (Remember that the charge of the conjugate base will be different by +1). The weaker conjugate acid will have the stronger base.
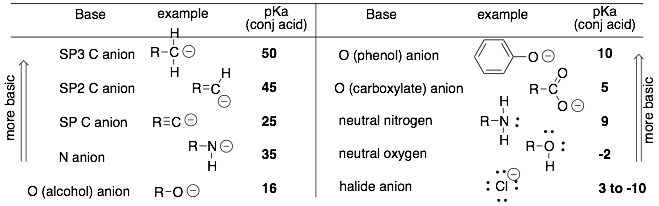
Hint: The basicity trends are opposite to that of the acidity trends. a) The more stable lone pair is less basic. b)The lone pair on the more EN is less basic (as long as you are comparing to atoms with the same charge).
Examples:
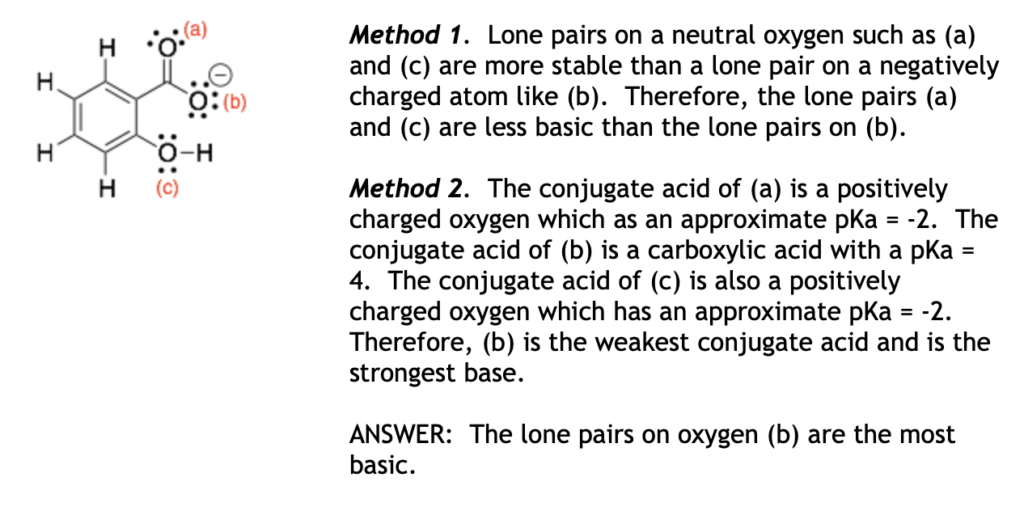
Hint: Lone pairs on negatively charged atoms are usually more basic than lone pairs on neutral atoms.


