Identify Chiral Centers
Introduction:
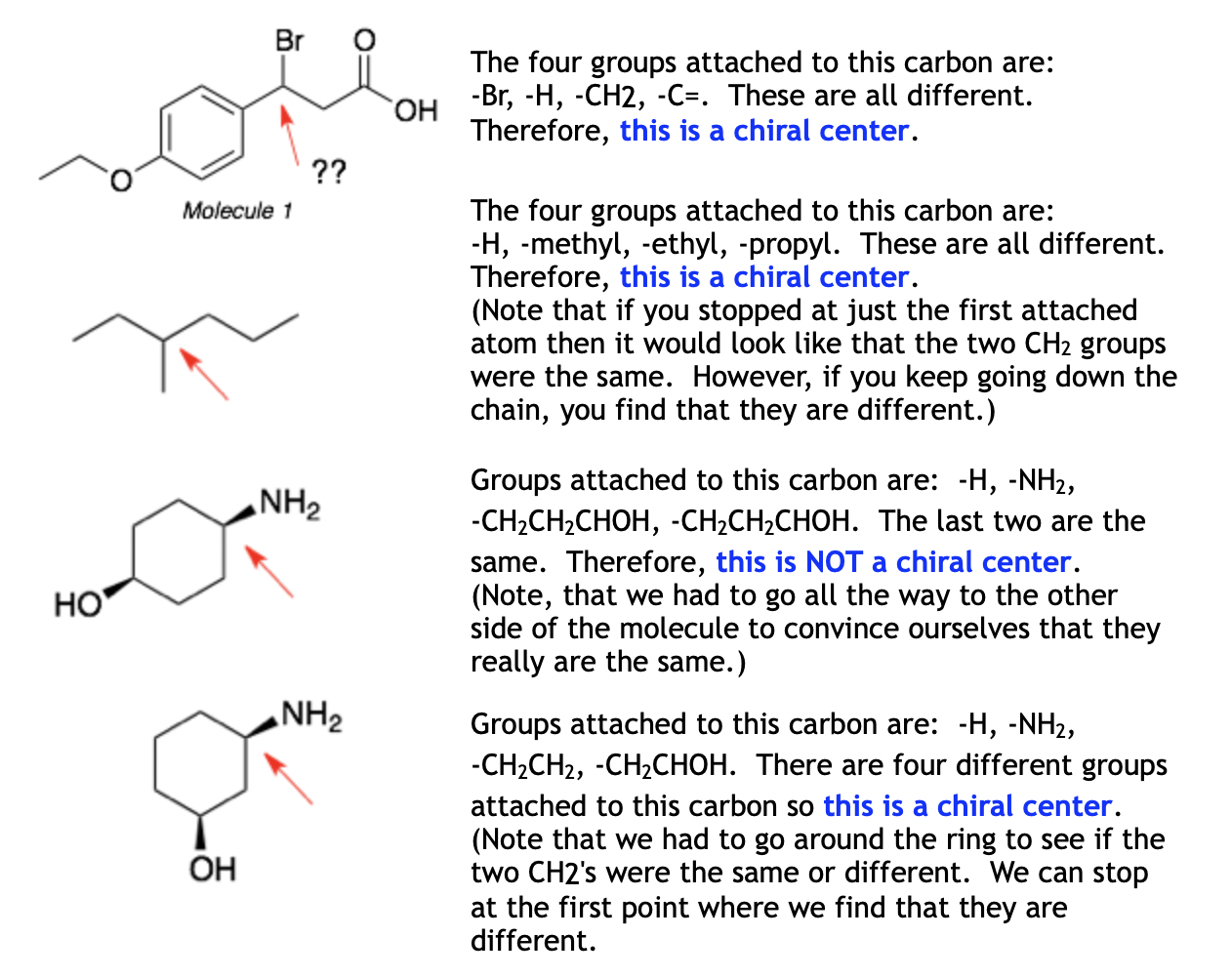
Chiral centers are tetrahedral atoms (usually carbons) that have four different substituents. Each chiral center in a molecule will be either R or S. As noted above, molecules with a single chiral center are chiral. Molecules with more than one chiral center are usually chiral. The exceptions are meso-compounds.
How to:
Step 1: Eliminate the atoms that cannot be chiral centers. These include CH2 groups, CH3 groups, oxygens, halogens, and any atom that is part of a double or triple bond.

Step 2: For the remaining atoms, list out the groups (substituents) attached to that atom. If there are four different groups, then it is a chiral center. (Note that two substituents can appear to be the same if you look only at the first attached atom but you have to keep going to check if they are really the same or are different.)