Identify Chiral Molecules
Introduction:
Chirality is a property of individual molecules. If a molecule is chiral then we know that the molecule has two enantiomeric forms (which are almost identical but are actually different molecules). I like to think of this as the ‘twin’ property. If a molecule is chiral then we know that it has a twin (an enantiomer). If a molecule is not chiral (achiral) then it is unique and does not have a twin. However unlike people, we can know if a molecule has a twin just by looking at one molecule.
How to:
There are three common tests for chirality. You can use which ever test is easiest to apply for a particular molecule. (Note: that they should all give the same answer).
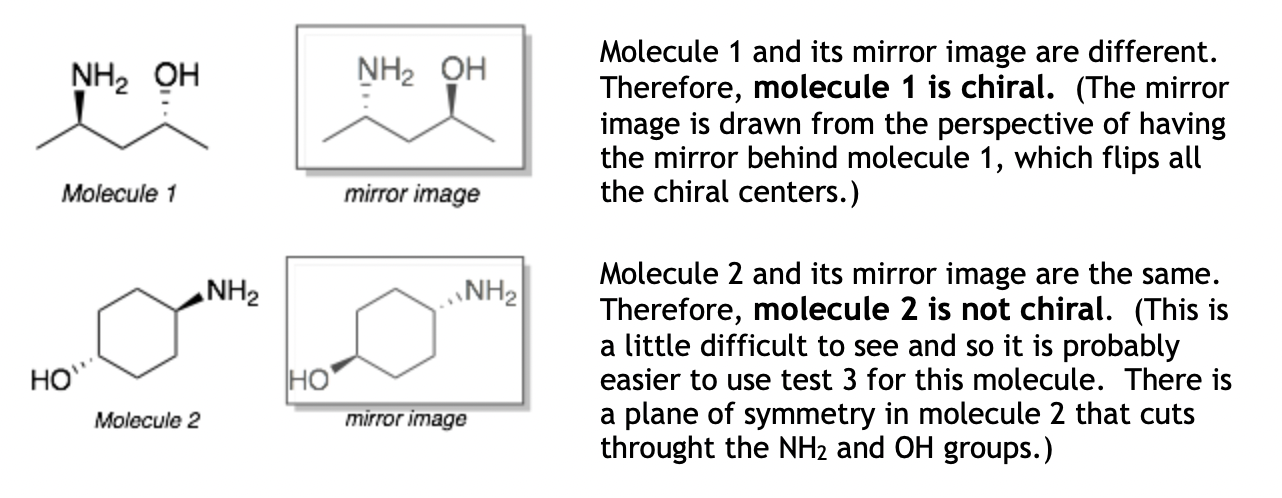
Chirality Test 1: Draw the mirror image of the molecule and see if the two molecules are the same or different. If they are different, then the molecule is chiral. If they are the same, then it is not chiral. (This is the most comprehensive test but is the most difficult to apply.)
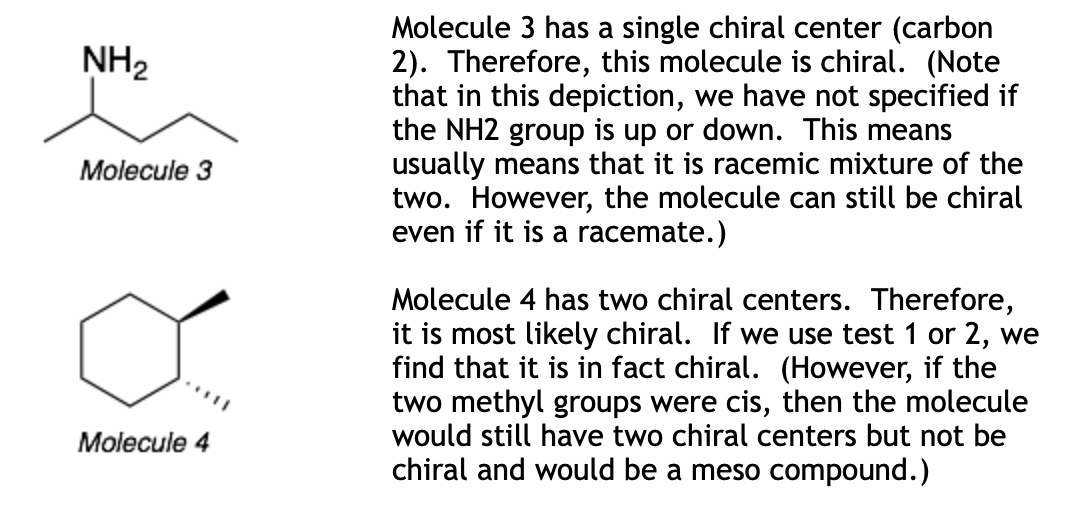
Chirality Test 2: If the molecule has only one chiral center, then the molecule is chiral. If the molecule has more than one chiral center, it is most likely chiral. The exceptions are meso molecules, which have chiral centers but are not chiral due to the presence of a plane of symmetry.
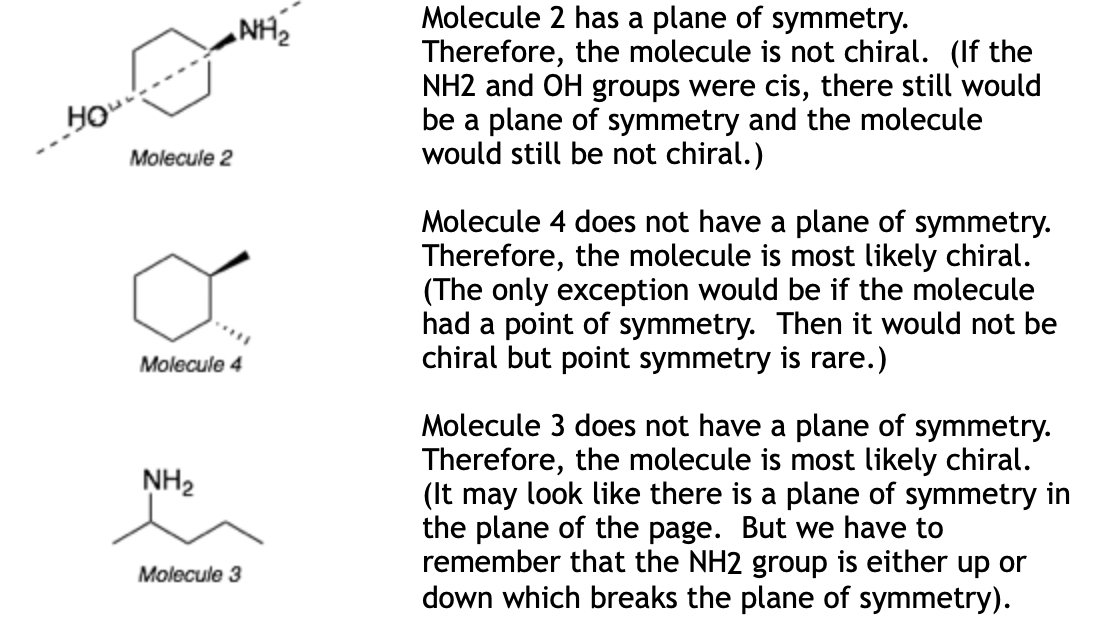
Chirality Test 3: If a molecule has a plane of symmetry, then the molecule is not chiral (achiral). (This is probably the easiest test to apply. But the symmetry can be hard to see sometimes depending on how the molecule is drawn.)