Identify functional groups
Introduction:
Each molecule has unique properties and reactivities. It is difficult to predict these for each new molecule. One way to simplify this process is to identify common arrangements of atoms (also known as functional groups) within a molecule. Functional groups typically have similar properties and reactivities and thus can provide a simple guide to predicting many molecular attributes. (Note: that most organic textbooks are arranged by functional groups for this reason).
How to:
1. Below are list of common functional groups with two or three examples for each
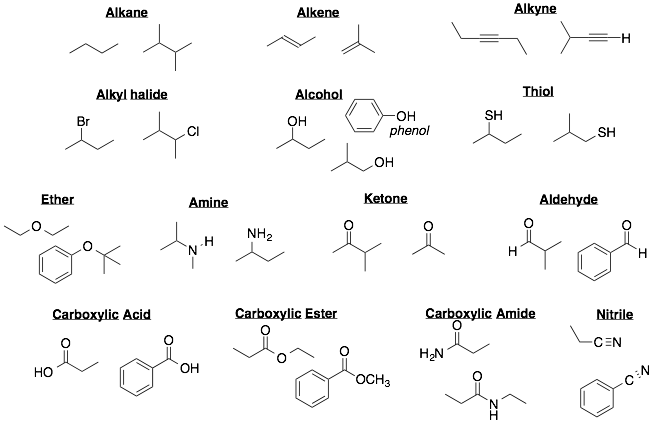
2. Some functional groups can be further classified as primary, secondary, tertiary, or quaternary (1°, 2°, 3°, or 4°). The rules for these subclassifications differ slightly for each functional group.
a) Alkanes. Each carbon in an alkane can be classified as 1°, 2°, 3°, or 4°. To do so, simply count the number of carbons attached to the carbon in question.
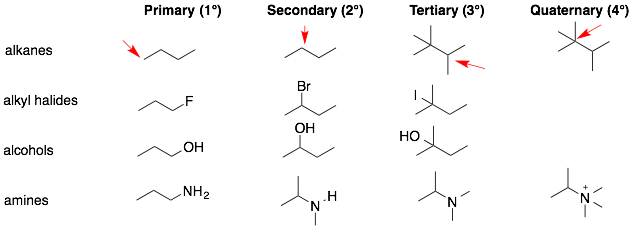
b) Alkyl halides and alcohols. For the remaining functional groups (alkyl halides, alcohols, amines), the functional group can be classified as 1°, 2°, 3°, or 4°. For alkyl halides and alcohols, the classification is based on the number of carbons attached to the carbon directly attached to the halide or OH group.
c) Amines. For amines, the classification rules are slightly different. Count the number of carbons directly attached to the amine group.