Make Flash Cards to Learn Reactions
Introduction
From the second exam onward, the remainder of the semester (and also next semester) will be dedicated to learning reactions. We will probably learn 20 to 30 new reactions for exam 3. This is a lot of information to learn quickly and so a good way to do this is using flash cards. Just the act of making flash cards can help organize the reactions in your head. More importantly, the flash cards actively test your memory of the reactions: backwards and forwards and in different orders. It also allows you to identify which reactions you are having trouble learning and allows you to focus on these reactions.
How to
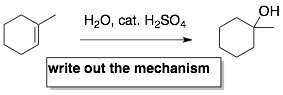
In each reaction, there are three pieces of information. These are the reactant, reagents, and product(s). In the example below, 1-methylcyclohexene is the reactant, water and catalytic sulfuric acid are the reagents, 1-methylcyclohex-1-ol is the product.
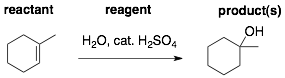
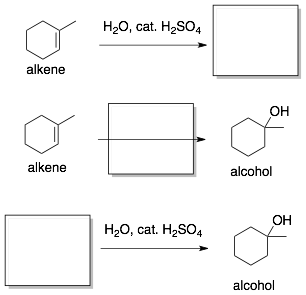
A typical question will be to provide you with two of these pieces of information and ask you for the third. You can practice for these types of questions using the flash cards. So, for the above reaction you would make the following three flash cards. In each case, the missing piece of information, which is highlighted by the box, would be on the back of the flash card.
The goal is to be able to quickly give the answers without going through the mechanisms. You will have to know the mechanism and this will be a separate type of question (and flash card). The reason for trying to avoid using the mechanisms for these types of questions is that it will slow you down and you will not be able to complete the exam. The analogy that I like to use is to think about learning reactions like learning multiplication tables. If you need to recalculate the answer each time (i.e. 5 x 5 = 5 + 5+ 5 + 5+ 5 +5), then it would take you forever to do long multiplication or division questions. The same is true for these reactions.
A couple of tips and comments:
- The easiest flash cards are the first two. The hardest is the last one. It requires knowing the reaction backwards and somehow, this is much more difficult. However, knowing the reaction ‘backwards’ will become very useful in the next section where we do synthesis problems that use multiple reactions to make a specific product.
- Note that below the reactants and the product, the key functional group being reacted or being formed are labled. This is important because the reactants and products on the exam will probably not look like the ones on your flash card. Therefore, if you just memorize the structures on your flash cards, you might not recognize the reaction. However, the functional groups being transformed will not change. So, in addition to writing out the structures of the answers, I would recommend that you fill in the missing functional group labels.
- When you practice with the flash cards, you should mix up the cards. As you work with the cards, you can start removing the ones that you feel comfortable with. That will allow you to focus on the ones that you are having trouble with.
- Since you are doing the above flash cards without the mechanisms. You need to make a separate (4th) card to learn the mechanism. This card will have all three pieces of information (reactant, reagents, and product) but will ask you to write out the mechanism. When you get this card, you need to get a sheet of paper and actually write out this the whole mechanism. So, it is usually everyones least favorite card. But, it is good practice. You will definitely have problems on the exam where you will have to write out the entire mechanism. If you just looked over the mechanisms in your notes or in your book, this is usually not enough.