Number of bonds and lone pairs
Introduction:
Atoms start with a specific number of valence electrons. They will then form bonds to try to fill up their valence shells. This leads to predictable numbers of bonds and non-bonding electrons because first and second row atoms cannot exceed a full shell.
How to:
(This method works for most atoms in the 1st and 2nd rows.* This includes the most common elements in Org Chem such as H, C, N, O, F, and halogens.)
The number of bonds for a neutral atom is equal to the number of electrons in the full valence shell (2 or 8 electrons) minus the number of valence electrons. This method works because each covalent bond that an atom forms adds another electron to an atom’s valence shell without changing its charge.
number of bonds for a neutral atom = (full valence shell) – (number of valence electrons)
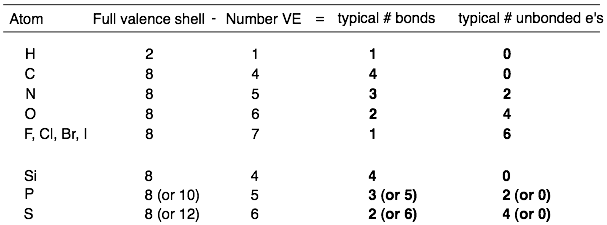
For example, hydrogen typically makes one bond because its full valence shell is 2 and its valence number is 1. Carbon typically makes four bonds because its full valence shell is 8 and its valence number is 4.
This same method can be used to calculate the number of electrons that are not participating in bonding. The number of non-bonding electrons is equal to the the number of electrons in a full valence shell minus the number of electrons which are participating in bonding (which is 2 x the typical number of bonds). The number of lone pairs is the number of non-bonding electrons divided by two.
number of non-bonding electrons for a neutral atom = (full valence shell) – 2 x (number of bonds)
For example, hydrogen typically has 0 non-bonding electrons. The full valence shell for hydrogen is 2 and the number of electrons in bonds is also 2. The difference is zero. Oxygen typically has 4 non-bonding electrons (or 2 lone pairs). The full valence shell for oxygen is 8 and the number of electrons in bonds is 4. Therefore, the difference is 4.
Table of the typical numbers of bonds and non-bonding electrons.
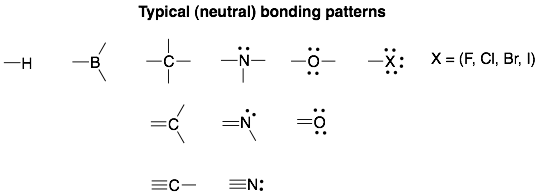
Advanced topics:
Because the third and fourth-row elements do not consistently follow the octet rule, it is difficult to predict their typical bonding patterns. The third-row elements Si, P, S usually follow the above rules and form 4, 3, and 2 bonds respectively. However, P and S can sometimes exceed the octet rule and make more than four bonds.
*Notice that boron is also missing from the above analysis. Boron cannot stay neutral while also completing its octet. Therefore, boron will stay neutral by forming three bonds but with an incomplete octet (with only 6 VEs). Alternatively, boron will fulfill its octet by forming four bonds but with a negative formal charge.