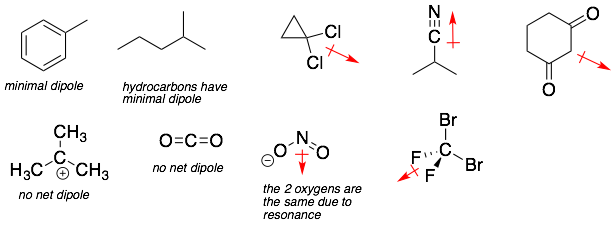
Predict if a molecule has a dipole moment
Introduction:
A molecular dipole is the sum of all the individual polarized bonds. If a molecule has a strong dipole moment, then it will form strong attractive interactions with other molecules with a dipole moment such as itself. This property is important for predicting many molecular properties such as boiling point, melting point, and solubilities.
Molecular dipoles are represented by an arrow with a bar. The direction of the arrow represents the orientation of the molecular dipole. The length of the arrow represents the magnitude of the molecular dipole.

How to:
These are challenging problems because they require you to be able to: 1) draw Lewis Structures with all the lone pairs from a molecular formula. 2) Identify polarized bonds. 3) predict the 3D geometry of the molecule. 4) Do a little qualitative vector math to see if the polarized bonds cancel.
To answer the question of whether a molecule has a dipole follow the instructions below:
- Generate the Lewis Structure with all of the lone pairs. In some problems, you will be given only molecular formula. In other problems, you will be given the Lewis Structure but usually without the lone pairs.
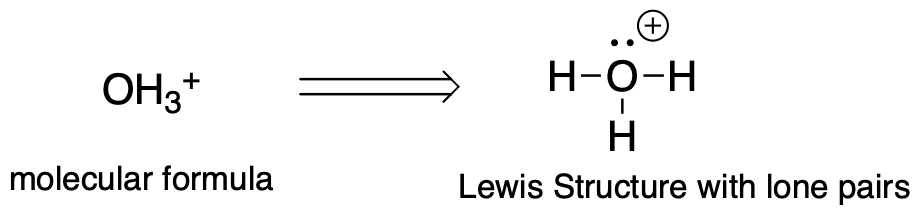
- Predict the geometry of the central atom or atoms of the molecule (using VSPR theory). The answer should be tetrahedral, trigonal planar, or linear. This is where it is important to have identified all of the lone pairs on the atoms.
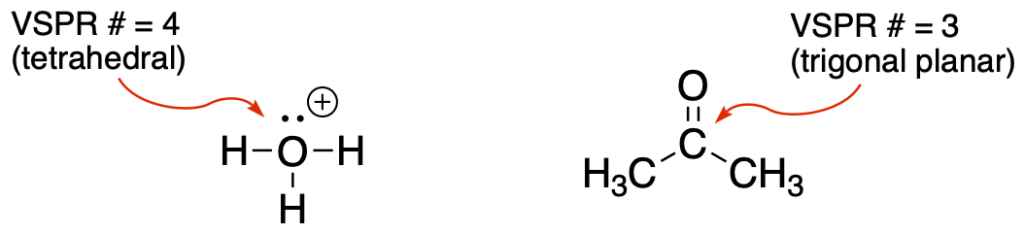
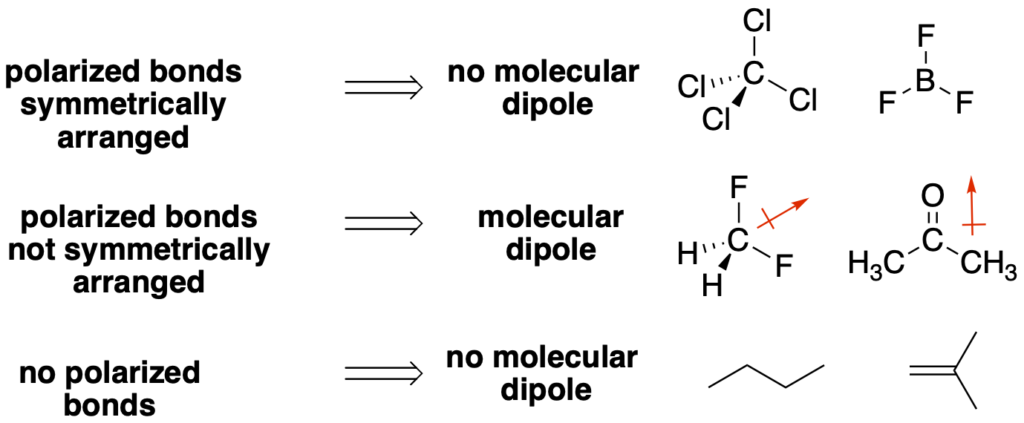
- Identify the polarized bonds in the molecule and draw an arrow for each bond in the direction of the more electronegative atom. These are bonds between atoms with very different electronegativies. Examples include: C-F, C=O, C-O, C-N, C-Cl, O-H, etc…
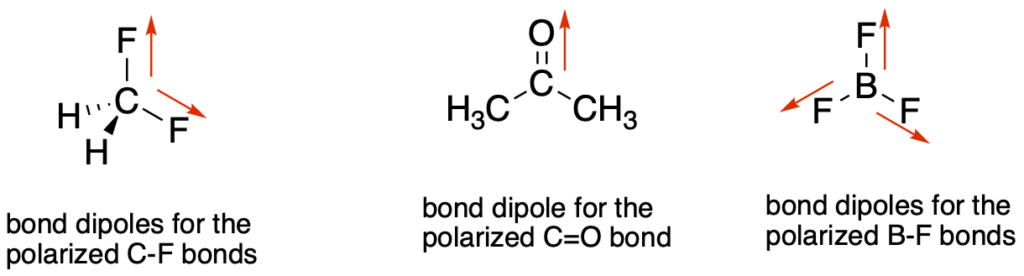
- Based on the geometry of the central atoms, are the bond dipoles arranged in a symmetrical orientation so that they cancel? If so, then there is no molecular dipole. If they do not cancel, then there is a molecular dipole. If there are no polarized bonds, then there is no molecular dipole.
HINTS:
•The carbon-hydrogen (C-H) and carbon-carbon (C-C) bonds can be generally ignored because they have little or no polarization. This is very helpful for organic molecules where these two bonds make up the highest percentage of bonds.
•For a molecule with a central tetrahedral atom, the only way symmetrically arrange polarized bonds is to have ALL four bonds to the central tetrahedral atom be the same (see the tetrahedral atoms below).

Examples: