Predict the 3D geometry and hybridization of a bonded atom
Introduction:
Lewis Structures are simple 2D models and do not make any predictions about the 3D structure of atoms. To do that we need to use the VSEPR (valence shell electron pair repulsion model).
How to:
In VSEPR (valence shell electron pair repulsion) theory, we count up the number of attached atoms and lone pairs and from this number we can predict the 3D geometry of an atom. (Note: do not count the number of bonds!) If an atom has:
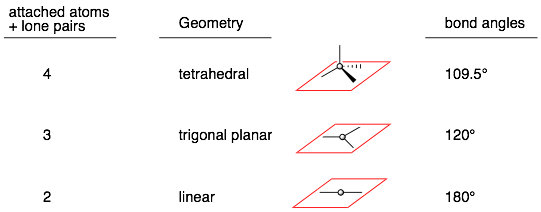
Example:
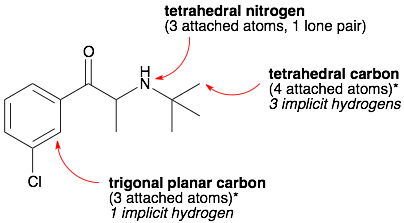
Comments: The bond angles are for an ideal system where all the groups attached to the central atom are the same but it is a pretty reasonable guess for other systems. For example in water, the central oxygen has two attached atoms (H’s) and two lone pairs for a total of four. Therefore, VSEPR theory predicts that water will be tetrahedral and that the H-O-H bond angle will be 109.5°, which is pretty close to the measured 105°. (Note, that this smaller angle suggests that lone pairs are “bigger” than H’s.)
Advanced Topics:
So, why does VSEPR theory work? As the repulsion part of the VESPR name implies, the predicted geometries are the ones that would result if 4, 3, and 2 clouds of electrons tried to get as far away as possible (repelling each other) while also still being tethered to a central atom.