Predict the electronegativity of an atom
Introduction:
Electronegativity is a measure of the attraction of an atom for an electron. The higher the electronegativity the greater the attraction of that atom for an electron. The electronegativity scale goes from 0 to 4.0 (for fluorine).
How to:
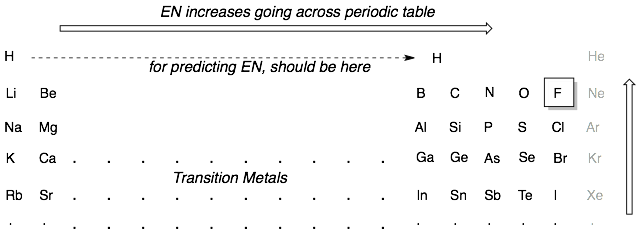
Fluorine is the most electronegative atom (EN = 4.0). Therefore atoms closer to fluorine will be more electronegative. The trend is for electronegativity to increase going across and up the periodic table.
Examples:
Oxygen is more electronegative than carbon. Chlorine is more electronegative than iodine. Sulfur is more electronegative than silicon.
Advanced topic:
A key exception to the above trends is hydrogen. Although we often put hydrogen all the way on the left hand side of the periodic table with the alkali earth metals such as Li and Na, the EN of hydrogen is much closer to the third column elements like boron. This means that C-H bonds are only weakly polarized because they have very similar electronegativities, with carbon being slightly more EN than hydrogen.