Predict the hybridization of an atom
Introduction:
Individual atoms have S and P orbitals. However, when atoms form bonds, the S and P orbitals “morph” into new hybrid orbitals with trajectories can that better accommodate the different bonding geometries.
How to:
The hybridized orbitals SP3, SP2, and SP are part S and part P.
- SP3 orbitals are 25% S and 75% P.
- SP2 orbitals are 33% S and 67% P.
- SP orbitals are 50% S and 50% P.
Hybridized orbitals look like P orbitals that have one lobe bigger than the other. SP3 orbitals are longer than SP2 because they have more P-character, and SP2 orbitals are longer than SP orbitals.
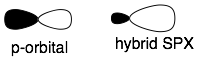
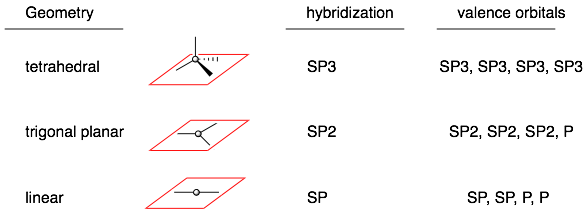
The hybridized orbitals point toward the attached atoms or lone pairs. In other words, they follow the black lines in the picture above. For example in an SP3 hybridized atom, the four SP3 orbitals all point outward in a tetrahedral geometry. In an SP hybridized atom, the two SP orbital point in opposite directions (180°). The P orbitals in SP and SP2 hybridized atoms are perpendicular to the hybridized orbitals.

Advanced Topics:
Sometimes a pair of resonance structures will predict different hybridizations. In these cases, choose the hybridization in this order SP > SP2 > SP3. For example in the case below, we would predict that the nitrogen was SP2 (trigonal planar) even though the resonance structure on the right is a minor structure.
