Predict the orbitals that form a bond
Introduction:
In organic chemistry, we focus on the two most basic types of bonds: sigma-bonds and pi-bonds. In a sigma bond, the electrons in the bond are found in the region directly between the two atoms. The electrons in a pi-bond follow arc shaped geometry (above and below) the two bonded atoms. Because of this shape, pi-bonds are sometimes called banana bonds.

How to:
Sigma- and pi-bonds are made from the overlap two orbitals (one on each atom)
- A single bond is a sigma-bond.
- A double bond is one sigma-bond and one pi-bond.
- A triple bond is one sigma-bond and two pi-bonds.
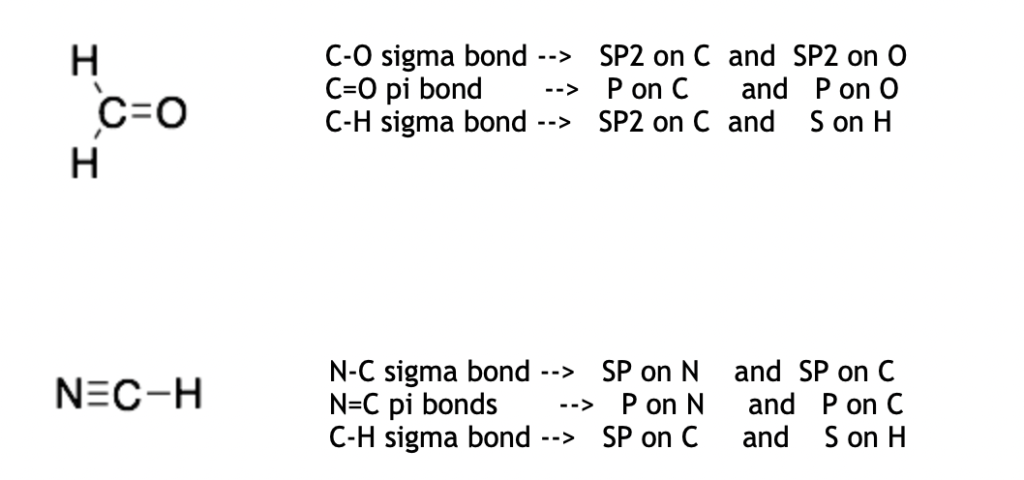
Sigma-bonds are made from the overlap of two hybridized orbitals (SP, SP2, or SP3). These will change depending upon the hybridization of the two atoms forming the sigma-bond.*
Hint: Pi-bonds are always made from two P-orbitals.
*Hydrogens do not have hybridized orbitals and will make sigma-bonds with their S-orbital. Hydrogens cannot make pi-bonds.
Examples:
Advanced Topics:
Sigma-bonds are formed from the hybridized orbitals on each atom. The two atoms forming a bond can have different hybridizations and therefore, the bond connecting the two atoms maybe formed from two different types of hybridized orbitals. For example in the molecule below, the C-C sigma-bond is formed from an SP2 orbital (on the left carbon) and an SP3 orbital (on the right carbon).
Practice Problems:
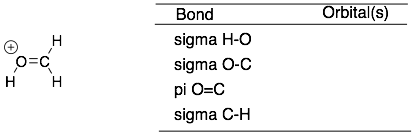
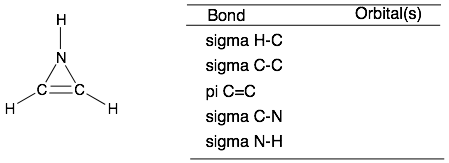
Scroll down for the answers
Answers:
(top structure) S and SP2, SP2 and SP2, P and P, SP2 and S
(bottom structure) S and SP2, SP2 and SP2, P and P, SP2 and SP3, SP3 and S