Predict the Regiochemistry (Markovnikov’s Rule)
Introduction:
In the reaction of alkenes where two different groups (A ≠ B) are added across a C=C, there are two possible products (as shown below), which are conformational isomers. Usually, there will be a strong preference for one constitutional isomer product over the other.
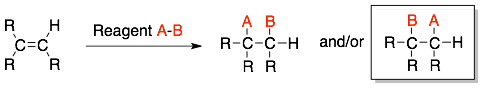
How to:
In the addition product, the less electronegative group (A) will be attached to the less substituted carbon of the alkene. Then, the more electronegative group (B) will be attached to more substituted alkene. This rule is often referred to as Markovnikov’s rule.
Below is a list of the A- and B- groups for the common alkene addition reactions. The less electronegative group is the A- group. The more electronegative group is the B- group. (When comparing electronegativities of groups with multiple atoms, pick the atom that is directly attached to the product.)
| reaction (reagent) | A- and B- groups |
| HX addition (HCl or HBr) | H and X (Br or Cl) |
| hydration (H3O+ or H2SO4, H2O) | H and OH |
| hydrobromination (Br2, H2O) | Br and OH |
| oxymercuration-reduction (1. HgOAc2, H2O 2. NaBH4, NaOH) | Hg(OAc) (H)1 and OH |
| hydroboration-oxidation (1. BH3, 2. NaOH, HOOH) | BH2 (OH)1 and H |
HINT: Hydrogen will usually be on the less substituted carbon of the C=C. This is the result of Markovnikov’s rule. Hydrogen is one of the least electronegative atoms and so it will usually be the less electronegative group.
Example 1
In the hydration reaction of 1-methyl-cyclohexene, the two groups added across the C=C are -H and -OH. The less electronegative group is -H and so it will be attached to the less substituted carbon of the alkene.
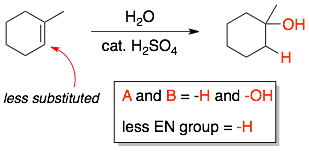
Example 2
In the HBr addition to 1-hexene, the two groups added across the C=C are -H and -Br. The less electronegative group is -H and so it will be attached to the less substituted carbon of the alkene.
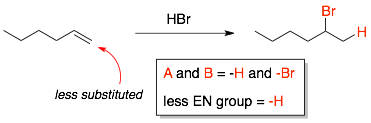
Example 3
In the hydroboration-oxidation reaction of 1-hexene, the two groups added across the C=C are -BH2 and -H. The less electronegative group is -BH2 (or -BR2). So, in the first addition reaction, the BH2 will be attached to the less substituted carbon of the alkene. In the second step of the reaction, the boron group is oxidized and transformed into an OH group.
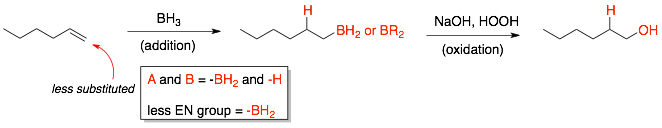
NOTE: The hydroboration-oxidation reaction is considered an anti-Markovnikov transformation because the more electronegative group (-OH) is on the less substituted carbon in the final product. However, the reaction actually follows Markovnikov’s rule for the addition reaction. In the first step, the less electronegative group (-BH2) is attached to the less substituted carbon. However, after the oxidation step, the more electronegative group (-OH) is attached to the less substituted carbon.