Predicting bond polarization
Introduction:
As noted on the previous page, when two atoms have very different electronegativities (ENs), they form ionic bonds. Conversely, when two atoms have very similar ENs they form covalent bonds. However, this is a sliding scale. So, most covalent bonds also have some ionic character. In these polar (or polarized) covalent bonds, one atom has a partial negative charge (∂-) and the second atom has a partial positive charge (∂+).
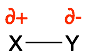
The direction of the bond polarization and the degree of polarization can be very helpful in predicting the properties (i.e. dipole) and reactivity of molecules.
How to:
Covalent bonds formed from two different elements will always have some bond polarization. The degree of bond polarization can be estimated from how far apart the two elements are on the periodic table. The atom closer to fluorine in the periodic table will bear the partial negative charge. Conversely, the atom further from fluorine will bear the partial positive charge.
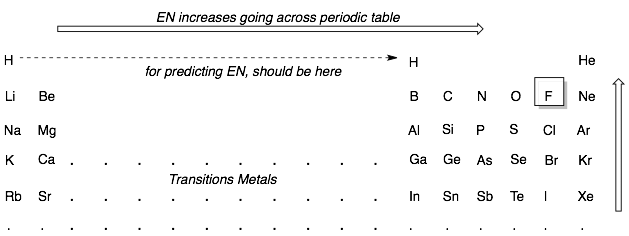
Examples:
The most important bonds in organic chemistry are those involving carbon. So, most of the examples will focus on bonds involving carbon.
C-C –> no polarization
C-H –> weak polarization (∂- on carbon and ∂+ on hydrogen)
C-N, C=N –> moderate polarization (∂+ on carbon and ∂- on nitrogen)
C-O or C=O –> strong polarization (∂+ on carbon and ∂- on oxygen)
C-X (X=F, Cl, Br, I) –> strong polarization (∂+ on carbon and ∂- on halogen)
H-X (X=F, Cl, Br, I) –> strong polarization (∂+ on hydrogen and ∂- on halogen)
H-B –> weak polarization (∂- on hydrogen and ∂- on boron)
Comments:
- Most bonds involving carbon will have a ∂+ on carbon because most common elements are more electronegative than carbon. The exception are C-H, C-B bond, and C-Si bonds.
- Hydrogen is also not very electronegative and so it usually makes bonds where the hydrogen is ∂+. One exception is the H-B bond, where hydrogen is slightly more electronegative than boron.
- At least to the first approximation, single, double, and triple bonds will not change the bond polarization. The electronegativities of the atoms in the bond is the most important factor in determining bond polarization.