Predict the number of valence electrons in an atom
Introduction:
The valence electrons (VE) are the electrons in the outer shell of an atom. The valence electrons are the ones involved in forming bonds to adjacent atoms. Therefore, the number of VE is important for determining the number of bonds an atom will form, the number of unpaired electrons, and an atom’s formal charge.
How to:
For neutral atoms, the number of valence electrons is equal to the atom’s main group number.
number of VE for neutral atom = main group number
The main group number for an element can be found from its column on the periodic table. For example, carbon is in group 4 and has 4 valence electrons. Oxygen is in group 6 and has 6 valence electrons.
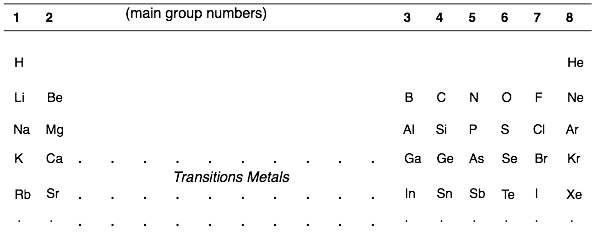
For charged atoms, there is a deviation from the normal number of valence electrons. The charge on an atom tells you how much of a deviation there is from an element’s normal valence number. Electrons are negatively charged. So a negatively charged atom means that there are more electrons than the normal valence number. A positively charged atom has fewer electrons than the normal valence number.
number of VE for charged atom = (main group number) – (charge)
Examples:
Ca+2 has zero valence electrons. Calcium normally has 2 valence electrons but with a 2+ charge it is missing two electrons. F-1 has 8 valence electrons. S-2 has 8 valence electrons.