Search for new topics by keyword
“Seeing” implicit hydrogens in abbreviated Lewis Structures
Introduction:
In organic chemistry, we often draw abbreviated Lewis Structures that do not have labels for the carbons and do not show hydrogens attached to carbons. This saves a lot of time and space as the majority of atoms in organic molecules are carbons and hydrogens. However, this can lead to confusion particularly for the “implicit” hydrogens which are not shown.
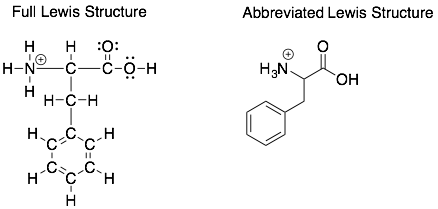
How to:
To identify the implicit hydrogens in an abbreviated Lewis Structure, try to follow the following rules.
- Only carbon atoms will have implicit hydrogens. The hydrogens to other atoms such as oxygen or nitrogen should be shown.
- Fill in the missing bonds for each carbon with bonds to hydrogens. Neutral carbons will have four bonds. Cationic and anionic carbons will have three bonds. Therefore, if the abbreviated structure only shows 2 bonds to a neutral carbon, then the carbon will be bonded to 2 implicit hydrogens.
Examples:

More examples
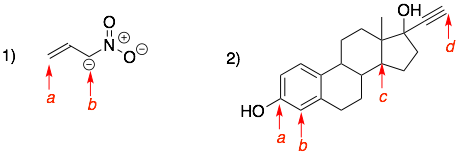
Answers: 1a) 2 hydrogens, 1b) 1 hydrogen, 2a) 0 hydrogens, 2b) 1 hydrogen, 2c) 1 hydrogen, 1d) 1 hydrogen