“Seeing” implicit lone pairs in Lewis Structures
Introduction:
In organic chemistry, we often draw abbreviated Lewis Structures that do not have labels for the carbons and do not show hydrogens attached to carbons. This saves a lot of time and space as the majority of atoms in organic molecules are carbons and hydrogens. However, it is often important to know where the ‘implicit’ lone pairs are to help predict the stability and reactivity of a molecule.
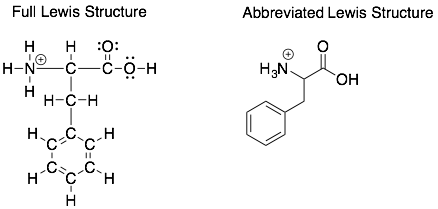
How to:
To identify the implicit lone pairs in an abbreviated Lewis Structure, follow the following rules.
- Assume that the formal charges are correct.
- For non-carbon atoms (such as nitrogen, oxygen, halogens etc), adjust the number of lone pairs to get the stated formal charge. See the table below for neutral, positively charged and negatively charged atoms.
- For carbon atoms, there are implicit hydrogens AND implicit lone pairs. Again, the easiest way to recognize the implicit lone pairs is to memorize the common configurations of carbon.
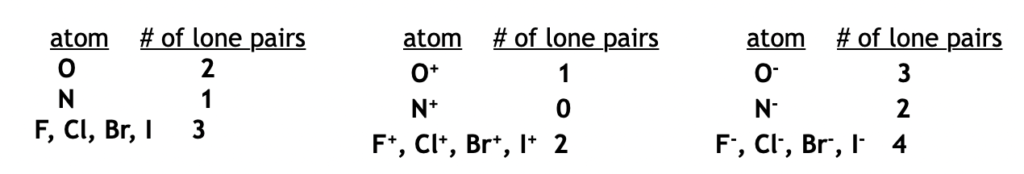
Hint: The most important atom in organic chemistry is carbon. So it is very useful to remember that a neutral carbon = 0 lone pairs, a negatively charged carbon = 1 lone pair, and a positively charged carbon = 0 lone pairs.
Examples:
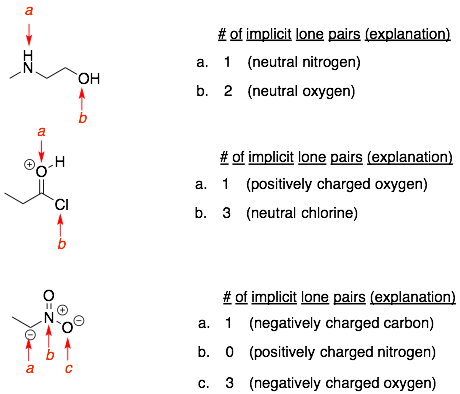
More examples:

Answers: 1a) 1 lone pair, 1b) 3 lone pairs, 1c) 2 lone pairs, 2a) 0 lone pairs, 2b) 1 lone pair, 2c) 1 lone pair.