Draw a tetrahedral carbon
Introduction:
One limitation of bond-line drawings is that they are 2D representations. For most organic molecules, these 2D drawings are reasonable representations. However, difficulties arise for molecules which contain tetrahedral atoms that have more than 2 (non-hydrogen) substituents. The 3rd and 4th substituents of a tetrahedral atom will not lie in the plane of the paper and so must be shown as coming out (bold wedge) or going into (dashed wedge) of the plane of the paper.

How to:
First, it is important to understand what a tetrahedron looks like and how it is generally depicted in 2D. For this exercise, we will use the example of dichloromethane (CH2Cl2). First build a model of dichloromethane using your model kit. If you do not have access to a model kit you can view an on-line 3D model of dichloromethane using this link:
http://www.chemspider.com/ImageView.aspx?mode=3d&id=6104
Hold your 3D model so it looks like the picture on the left, below. Note how the H, C, H atoms form a plane. This will be the plane of the paper. One chlorine is coming out of the plane and one chlorine is going into the plane.
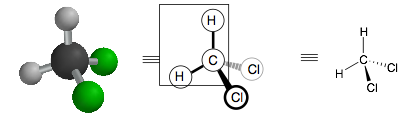
To depict this molecule in 2D, we draw the two C-H bonds as normal single bonds (that are in the plane of the paper). Then one chlorine has a bold wedge (coming out of the plane of the paper). The other has a dashed wedge (going into the plane of the paper.)
Second, we can depict the same molecule in 2D where different atoms have bold or dashed wedged bonds. These represent different views of the 3D structure. For example, now hold your 3D model so it looks like the picture below. Now, the Cl, C, Cl atoms form a plane, which will be the plane of the paper. The two hydrogens now come in and out of the plane and so are depicted as bold and dashed wedges.
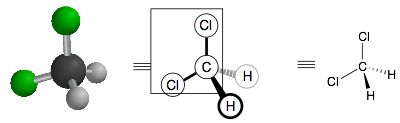
Below are some more 2D depictions of dichloromethane. Note, that these are all the same molecule. They are simply 2D views of the tetrahedron from different angles. Using your 3D model, rotate the model so that it matches up with each of these 2D representations to convince yourself that these are really all the same molecule. The difference is that the three atoms that define the plane of the page are different in each one.
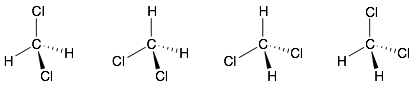
Summary:
In the 2D representations of tetrahedral atoms, two of the groups and the center atom will be in the plane of the paper. (Remember, three points makes a plane.) The remaining two groups will be coming in and out of the plane.