Predict the pKa of a molecule
Introduction:
We learned a list of pKa’s (shown below). This subset of molecules was selected to:
1) Provide examples of the acidity trends that we learned such as electronegativity, atom size, resonance, inductance, atom hybridization. For example, the acidity trend of acetylene > ethylene > ethane shows an example of the hybridization trends where SP hybridized atoms are more acidic than SP2, which are more acidic than SP3.
2) The list can be used to estimate pKa’s of molecules. (Which is the subject of this handout.)
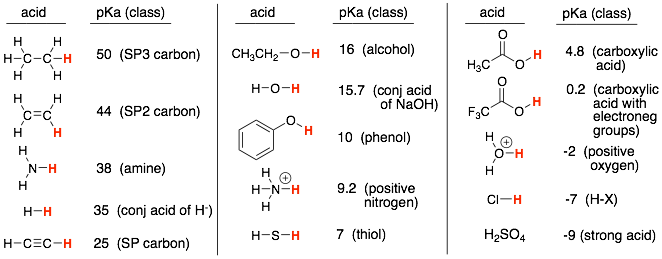
How to:
Each acid in the list is representative of common functional groups. These are shown in parentheses above. For example, ethanol (CH3CH2OH) is representative of alcohols (ROH), and ammonia (NH3) is representative of amines (RNH2, R2NH).
Therefore, identify the functional groups in the molecule and then match up these functional groups to the pKa’s in the table above.