Predicting boiling and melting points
Introduction:
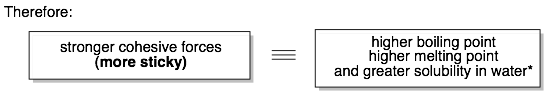
Many of the properties of organic molecules can be predicted based on the strength of their cohesive molecule-molecule interactions (i.e. how much the molecules want to stick together). The boiling point (BP) and melting point (MP) of an organic molecule is related to: 1) the molecular weight of the molecule and 2) the “stickiness” of individual molecules for their neighbors.
For example, molecules in a liquid are held together by attractive interactions between molecules. These interactions must be broken (by adding heat) in order to transition into the gas. The stronger the cohesive interactions, the more heat that is necessary for the liquid-to-gas phase transition. These same attractive molecule-molecule interactions have to be broken for a solid to liquid phase change.

Introduction to intermolecular forces:
All of the attractive intermolecular forces are based on the electrostatic attraction of positive charges on one molecule with negative changes. The stronger the charges, the stronger the attraction between molecules.

How to:
In general, comparisons can only be made between molecules with similar formulas or molecular weights.* First, identify the functional groups in the molecules being compared. Each functional group can form different types of intermolecular interactions (see the chart above). Interactions higher up on the chart form stronger cohesive interactions and will have higher boiling points and melting points.
Advanced topics
Larger molecules usually have higher boiling points and melting points. This is not because of their higher molecular weights but because they usually have greater surface areas with which to form more intermolecular interactions. This is also why linear molecules tend to have higher MPs and BPs than branched (or more spherically shaped) molecules. Linear molecules will have more surface area than a more spherically shaped molecule.
Examples:
(Rank the following molecules in terms of MP and BP.)

All three molecules have approximately the same molecular formula and molecular weights. Only molecule A contains a strongly polarized or ionic functional group: an aldehyde. The C=O group in aldehydes generate a strong molecular dipole (partial + and – charges). Thus, it can form weak attractive dipole-dipole interactions, and A will have the highest boiling point and highest melting point in this series of compounds.
Molecules B and C are alkanes. They can only form the weakest attractive dispersion interactions between their molecular surfaces. Due to its long-thin shape, B will have a greater surface area and can form more dispersion interactions than the rounder C. Therefore, B will have a higher boiling point and melting point than C.
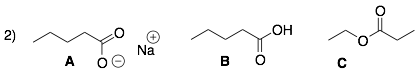
Molecule A is ionic (it has charges). Therefore, it can form the strongest ion-ion interactions and will have the highest boiling point and melting point.
Molecule B is a carboxylic acid with an acidic proton and basic lone pairs. Therefore, it can form medium strength hydrogen bonding interactions, and it will have the next highest boiling point and melting point.
Molecule C is a carboxylic ester, which is a polar functional group. However, it does not have an acidic proton. Therefore, esters cannot form hydrogen bonds with themselves. Instead, they can only form weak dipole-dipole interactions and will have the lowest boiling point and melting point in this series of compounds.