Search for new topics by keyword
Recognizing resonance structures
Introduction:
Resonance structures are multiple Lewis Structures that collectively describe a single molecule. Resonance structures are necessary to describe molecules that contain fractional bonds and fractional charges. This is much more common than one might expect as most covalent bonds have some ionic character and so are less than a full covalent bond (bond order < 1.0).
Resonance structures are different depictions of the same molecule, similar to the relationship between a front and profile picture of the same person. Resonance structures do not “resonate” or flip back and forth as the name implies.
How to:
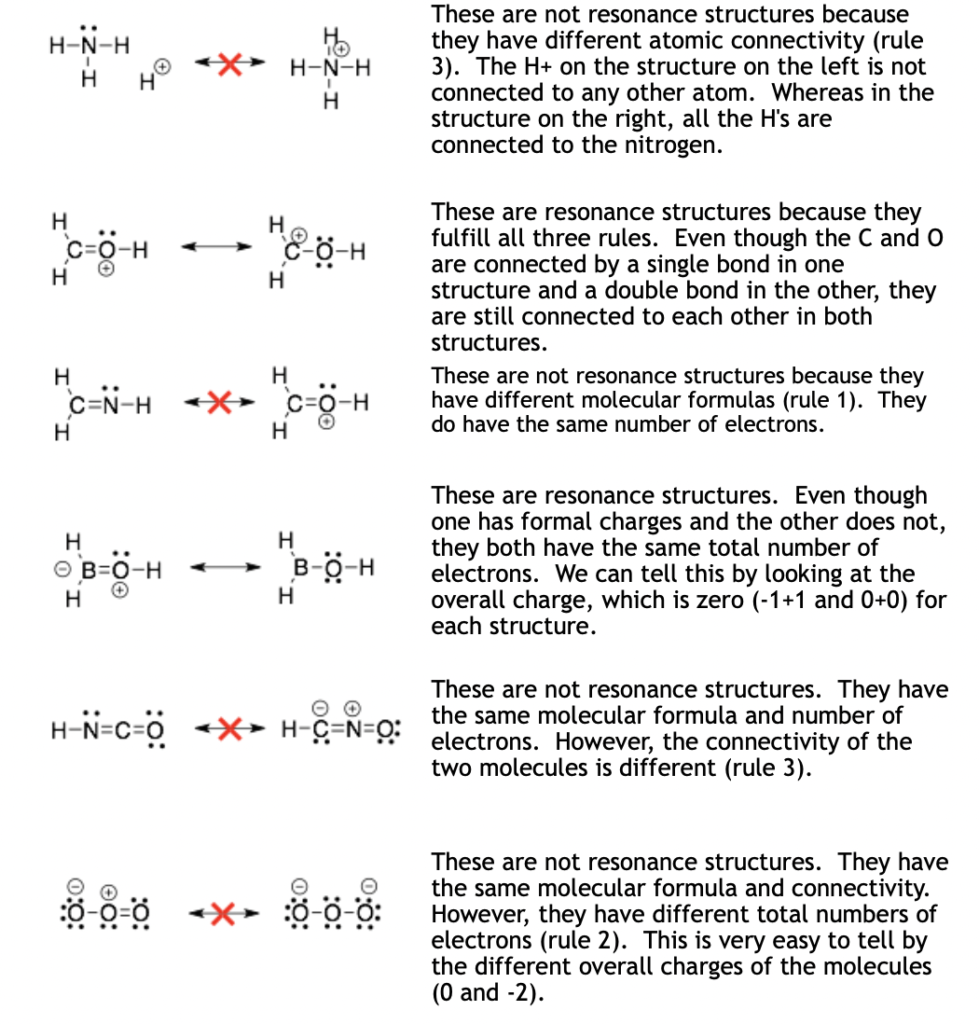
Because resonance structures are the same molecules, they must have:
- The same molecular formulas.
- The same total number of electrons (same overall charge).
- The same atoms connected together. Although, they can differ in whether the connections are single, double or triple bonds.
Examples: