Classify Nucleophiles and Bases (strong or weak)
Introduction:
The most important variable in deciding between SN1, SN2, E1, and E2 mechanisms is the structure of the alkyl halide (R-X). However, the second deciding variable is the strength of the nucleophile/base. I have tried to simplify this decision by putting all the nuc/bases into one of four categories. I have also ignored solvent effects which can change and complicate these reactivity trends.
How to:
1) Nuc/Base Strengths. The easiest categories to recognize are the strong/strong and weak/weak categories. These follow the general correlation between basicity and nucleophilicity. The more difficult categories are the weak/strong and strong/weak because they deviate from this correlation.
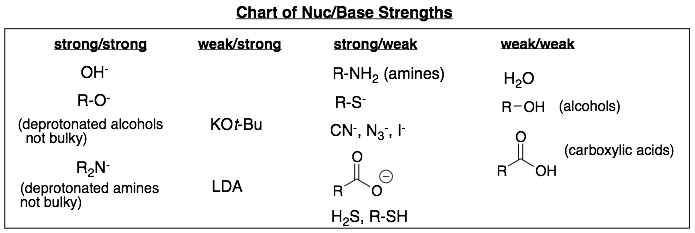
2) Descriptions and explanations of the four categories.
(i) Strong/strong. In general, good bases are also good nucleophiles. Therefore, strong bases such as negatively charged oxygens and nitrogens will also be strong nucleophiles.
Note, not all negatively charged oxygen and nitrogen nuc/bases fall into the strong/strong category. These exceptions populate the weak/strong and strong/weak categories.
(ii) Weak/weak. In general, weak bases are also weak nucleophiles. Therefore, weak bases such as neutral oxygens with a proton will also be weak nucleophiles.
Weak/weak nuc/bases are usually also the solvent for their reactions. This makes sense as they are so weak that you need a lot of the nuc/base to facilite the substitution or elimination reaction.
(iii) Weak/strong. One exception to strong bases also being strong nucleophiles is for very bulky nuc/bases. SN2 reactions are particularly sensitive to the size of the nuc/base because they proceed via a crowded transition state. Elimination reactions are less sensitive to the size of the nuc/base since the beta-hydrogen is sticking out and is easy to access.
Therefore, a very bulky (large) nuc/base can be a weak nucleophile while still being a strong base. We will only learn two nuc/bases that fall into this category. They are potassium tert-butoxide (KOt-Bu) and lithium diisopropyl amide (LDA). The structures of these two weak/strong nuc/bases are shown below.
examples of weak bulky nucleophiles that are strong bases (weak/strong)

(iv) Strong/weak. These nuc/bases fall into two general categories that will reduce their basicity:
- Neutral nuc/bases that have lone pairs on less electronegative atomssuch as nitrogen, sulfur, and phosphorous. These include amines, thiols and phosphines.
- Negatively charged nuc/bases that are stabilized by resonance or have a negative charge on a large atom such as sulfur or iodine.