Drawing and Interpreting Reaction Coordinate Diagrams
Introduction:
Another way describe a reaction mechanism is to plot the change in energy along the reaction pathway. This is shown in an Energy vs Reaction Coordinate plot.
In these plots, the y-axis is energy (with lower E being more stable). The x-axis is the reaction coordinate, which is a plot of the course of the reaction (from start to end).
Reaction coordinate plots are very useful in showing: 1) whether a reaction is an exo- or endothermic process, 2) which reaction pathway is faster, and 3) which products are the kinetic (fastest formed) and thermodynamic (most stable) products.
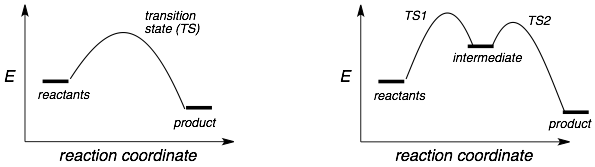
Key Features:
Each step in a mechanism will have a barrier or “hill”. The examples above show a one-step (left) and a two-step (rights) reaction respectively as they have they have one and two “hills”.
Rates (speed) of reactions: The rate of a reaction can be estimated by the height of the barrier (relative to the reactants). The higher the barrier… the slower the reaction. I like to think of these plots as if you were a mountain climber moving along the reaction path from left to right.
The highest “hill” in a reaction (relative to the reactants) is the slowest step. This slow step is also known as the rate determining step because a reaction will only go as fast as its slowest step.
Transition states: These are “tops of hills”. TS are very unstable species with partially formed and broken bonds. TS are very hard to capture and study. Therefore we do not know much about their relative stabilities and we often can only make systematic guesses. Note that a reaction can have multiple TS’s.
Intermediates. Intermediates are the “valleys” in between two hills. Intermediates have fully formed bonds but are always less stable than the reactants or products. Intermediates are often carbocations, carbanions, or radicals, which we know a lot about their stability trends.
Endothermic and exothermic. These terms refer to the overall energy change in a reaction. If a reaction is endothermic than it adsorbs energy. If a reaction is exothermic it gives off energy. Therefore, an endothermic reaction will have a product that is higher in energy than the reactants (because it adsorbed energy). Exothermic reactions will have products that are lower in energy than the reactants. Both of the reactions above are exothermic.