Substitution Reaction Overview and Traits
Introduction:
The first reaction that we introduce is usually the substitution reactions of alkyl halides (R-X). Because this is the first reaction, we often go into extricating details about every aspect of this reaction.
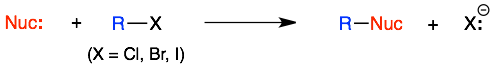
All of this information can be overwhelming. Not only is it hard to keep straight but some of it seems provide conflicting predictions (which it does). So this overview of some of the key traits of substitution reactions is provided.
Mechanisms:
There are two general substitution reactions (shown below) are SN2 and SN1. Mechanism are the step-by-step process that bonds are formed and broken. The number of steps and the order of steps in a mechanism is very important.
SN2 mechanism
This is a one-step mechanism. The nucleophile forms a bond to the electrophile (R) at the same time as the leaving group (X).

Comments: The key step is the formation of the crowded transition state (TS) shown in brackets. The carbon on R has five bonds! The bonds to both the nucleophile and leaving group are still partially formed/broken. This occurs from opposite sides of the R-group. The nucleophile needs to be strong because it must forcibly eject the nucleophile.
SN1 mechanism
This is a two-step mechanism. The R-X bond first breaks. This step is slow. Then the nucleophile forms a bond to the carbocation intermediate (R+).

Comments: The key step is the formation of the positively charged R+ intermediate. So, R will be 3° or 2° to form more stable carbocation intermediates. The carbocation intermediate is flat. So, the nucleophile can attack from either face. The nucleophile is weak (and usually neutral and protic) because it is not involved in the key step. (If the nucleophile is strong, then other mechanisms occur: SN2 or E2.)
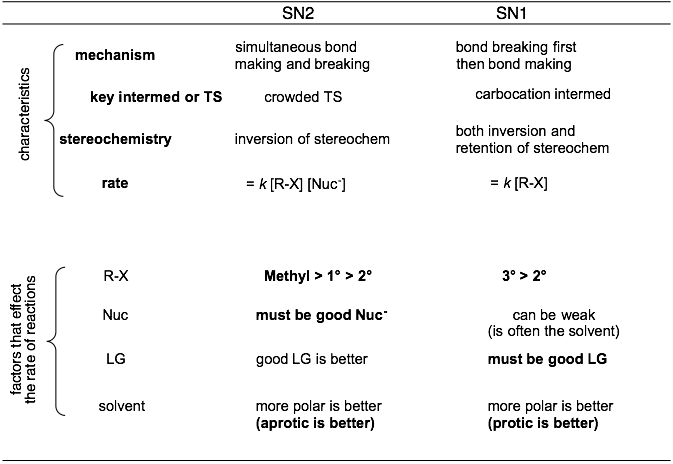
Summary of key traits: